Persistent coxsackievirus B infection and pathogenesis of type 1 diabetes mellitus
- PMID: 35650334
- PMCID: PMC9157043
- DOI: 10.1038/s41574-022-00688-1
Persistent coxsackievirus B infection and pathogenesis of type 1 diabetes mellitus
Abstract
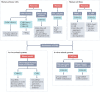
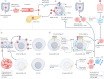
Enteroviruses are believed to trigger or accelerate islet autoimmunity in genetically susceptible individuals, thereby resulting in loss of functional insulin-producing β-cells and type 1 diabetes mellitus (T1DM). Although enteroviruses are primarily involved in acute and lytic infections in vitro and in vivo, they can also establish a persistent infection. Prospective epidemiological studies have strongly associated the persistence of enteroviruses, especially coxsackievirus B (CVB), with the appearance of islet autoantibodies and an increased risk of T1DM. CVB can persist in pancreatic ductal and β-cells, which leads to structural or functional alterations of these cells, and to a chronic inflammatory response that promotes recruitment and activation of pre-existing autoreactive T cells and β-cell autoimmune destruction. CVB persistence in other sites, such as the intestine, blood cells and thymus, has been described; these sites could serve as a reservoir for infection or reinfection of the pancreas, and this persistence could have a role in the disturbance of tolerance to β-cells. This Review addresses the involvement of persistent enterovirus infection in triggering islet autoimmunity and T1DM, as well as current strategies to control enterovirus infections for preventing or reducing the risk of T1DM onset.
© 2022. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

