A tissue-like neurotransmitter sensor for the brain and gut
- PMID: 35650358
- PMCID: PMC9210986
- DOI: 10.1038/s41586-022-04615-2
A tissue-like neurotransmitter sensor for the brain and gut
Abstract
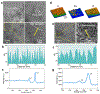
Neurotransmitters play essential roles in regulating neural circuit dynamics both in the central nervous system as well as at the peripheral, including the gastrointestinal tract1-3. Their real-time monitoring will offer critical information for understanding neural function and diagnosing disease1-3. However, bioelectronic tools to monitor the dynamics of neurotransmitters in vivo, especially in the enteric nervous systems, are underdeveloped. This is mainly owing to the limited availability of biosensing tools that are capable of examining soft, complex and actively moving organs. Here we introduce a tissue-mimicking, stretchable, neurochemical biological interface termed NeuroString, which is prepared by laser patterning of a metal-complexed polyimide into an interconnected graphene/nanoparticle network embedded in an elastomer. NeuroString sensors allow chronic in vivo real-time, multichannel and multiplexed monoamine sensing in the brain of behaving mouse, as well as measuring serotonin dynamics in the gut without undesired stimulations and perturbing peristaltic movements. The described elastic and conformable biosensing interface has broad potential for studying the impact of neurotransmitters on gut microbes, brain-gut communication and may ultimately be extended to biomolecular sensing in other soft organs across the body.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Figures









References
-
- Wise RA Dopamine, learning and motivation. Nature Reviews Neuroscience vol. 5 483–494 (2004). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

