Discovery of non-squalene triterpenes
- PMID: 35650436
- PMCID: PMC9177416
- DOI: 10.1038/s41586-022-04773-3
Discovery of non-squalene triterpenes
Abstract
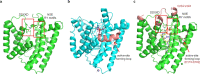
All known triterpenes are generated by triterpene synthases (TrTSs) from squalene or oxidosqualene1. This approach is fundamentally different from the biosynthesis of short-chain (C10-C25) terpenes that are formed from polyisoprenyl diphosphates2-4. In this study, two fungal chimeric class I TrTSs, Talaromyces verruculosus talaropentaene synthase (TvTS) and Macrophomina phaseolina macrophomene synthase (MpMS), were characterized. Both enzymes use dimethylallyl diphosphate and isopentenyl diphosphate or hexaprenyl diphosphate as substrates, representing the first examples, to our knowledge, of non-squalene-dependent triterpene biosynthesis. The cyclization mechanisms of TvTS and MpMS and the absolute configurations of their products were investigated in isotopic labelling experiments. Structural analyses of the terpene cyclase domain of TvTS and full-length MpMS provide detailed insights into their catalytic mechanisms. An AlphaFold2-based screening platform was developed to mine a third TrTS, Colletotrichum gloeosporioides colleterpenol synthase (CgCS). Our findings identify a new enzymatic mechanism for the biosynthesis of triterpenes and enhance understanding of terpene biosynthesis in nature.
© 2022. The Author(s).
Conflict of interest statement
T.L. has filed a patent application relating to the function of TvTS, MpMS and CgCS and the resulting structures talaropentaene and colleterpenol. The other authors declare no competing interests.
Figures






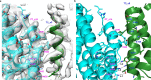


Comment in
-
A new path for terpenoid biosynthesis.Trends Biochem Sci. 2022 Nov;47(11):906-908. doi: 10.1016/j.tibs.2022.07.004. Epub 2022 Jul 29. Trends Biochem Sci. 2022. PMID: 35914998
References
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

