Combination anti-HIV antibodies provide sustained virological suppression
- PMID: 35650437
- PMCID: PMC11059968
- DOI: 10.1038/s41586-022-04797-9
Combination anti-HIV antibodies provide sustained virological suppression
Abstract
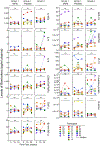
Antiretroviral therapy is highly effective in suppressing human immunodeficiency virus (HIV)1. However, eradication of the virus in individuals with HIV has not been possible to date2. Given that HIV suppression requires life-long antiretroviral therapy, predominantly on a daily basis, there is a need to develop clinically effective alternatives that use long-acting antiviral agents to inhibit viral replication3. Here we report the results of a two-component clinical trial involving the passive transfer of two HIV-specific broadly neutralizing monoclonal antibodies, 3BNC117 and 10-1074. The first component was a randomized, double-blind, placebo-controlled trial that enrolled participants who initiated antiretroviral therapy during the acute/early phase of HIV infection. The second component was an open-label single-arm trial that enrolled individuals with viraemic control who were naive to antiretroviral therapy. Up to 8 infusions of 3BNC117 and 10-1074, administered over a period of 24 weeks, were well tolerated without any serious adverse events related to the infusions. Compared with the placebo, the combination broadly neutralizing monoclonal antibodies maintained complete suppression of plasma viraemia (for up to 43 weeks) after analytical treatment interruption, provided that no antibody-resistant HIV was detected at the baseline in the study participants. Similarly, potent HIV suppression was seen in the antiretroviral-therapy-naive study participants with viraemia carrying sensitive virus at the baseline. Our data demonstrate that combination therapy with broadly neutralizing monoclonal antibodies can provide long-term virological suppression without antiretroviral therapy in individuals with HIV, and our experience offers guidance for future clinical trials involving next-generation antibodies with long half-lives.
© 2022. This is a U.S. government work and not under copyright protection in the U.S.; foreign copyright protection may apply.
Conflict of interest statement
Competing Interests Statement
There are patents on 3BNC117 (PTC/US2012/038400) and 10–1074 (PTC/US2013/065696) that list M.C.N. as an inventor. 3BNC117 and 10–1074 are licensed to Gilead Sciences by Rockefeller University from which M.C.N. has received payments. M.C.N. is a member of the Scientific Advisory Boards of Celldex Therapeutics, Walking Fish Therapeutics, and Frontier Biotechnologies. M.C.N. had no control over the direction and ultimately the reporting of the clinical portion of the research while holding their financial interests. All other authors declare no competing financial interests.
Figures







Comment in
-
Long-term control of HIV.Nat Rev Microbiol. 2022 Aug;20(8):446. doi: 10.1038/s41579-022-00759-0. Nat Rev Microbiol. 2022. PMID: 35705741 No abstract available.
-
Combination anti-HIV antibodies to achieve antiretroviral therapy-free virological suppression in infected individuals.Clin Transl Med. 2022 Sep;12(9):e1057. doi: 10.1002/ctm2.1057. Clin Transl Med. 2022. PMID: 36101965 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

