Molecularly defined circuits for cardiovascular and cardiopulmonary control
- PMID: 35650438
- PMCID: PMC9297035
- DOI: 10.1038/s41586-022-04760-8
Molecularly defined circuits for cardiovascular and cardiopulmonary control
Abstract
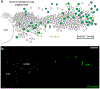
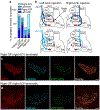
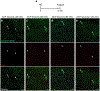
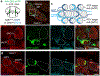
The sympathetic and parasympathetic nervous systems regulate the activities of internal organs1, but the molecular and functional diversity of their constituent neurons and circuits remains largely unknown. Here we use retrograde neuronal tracing, single-cell RNA sequencing, optogenetics and physiological experiments to dissect the cardiac parasympathetic control circuit in mice. We show that cardiac-innervating neurons in the brainstem nucleus ambiguus (Amb) are comprised of two molecularly, anatomically and functionally distinct subtypes. The first, which we call ambiguus cardiovascular (ACV) neurons (approximately 35 neurons per Amb), define the classical cardiac parasympathetic circuit. They selectively innervate a subset of cardiac parasympathetic ganglion neurons and mediate the baroreceptor reflex, slowing heart rate and atrioventricular node conduction in response to increased blood pressure. The other, ambiguus cardiopulmonary (ACP) neurons (approximately 15 neurons per Amb) innervate cardiac ganglion neurons intermingled with and functionally indistinguishable from those innervated by ACV neurons. ACP neurons also innervate most or all lung parasympathetic ganglion neurons-clonal labelling shows that individual ACP neurons innervate both organs. ACP neurons mediate the dive reflex, the simultaneous bradycardia and bronchoconstriction that follows water immersion. Thus, parasympathetic control of the heart is organized into two parallel circuits, one that selectively controls cardiac function (ACV circuit) and another that coordinates cardiac and pulmonary function (ACP circuit). This new understanding of cardiac control has implications for treating cardiac and pulmonary diseases and for elucidating the control and coordination circuits of other organs.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing Interests
The authors declare no competing interests.
Figures












Comment in
-
The cardiac parasympathetic system has two parallel circuits.Nat Rev Cardiol. 2022 Aug;19(8):503. doi: 10.1038/s41569-022-00742-5. Nat Rev Cardiol. 2022. PMID: 35761026 No abstract available.
References
-
- Langley JN The autonomic nervous system. (W. Heffer & Sons, Ltd., 1921).
-
- Palma JA & Benarroch EE Neural control of the heart: recent concepts and clinical correlations. Neurology 83, 261–271 (2014). - PubMed
-
- Appel ML, Berger RD, Saul JP, Smith JM & Cohen RJ Beat to beat variability in cardiovascular variables: noise or music? J. Am. Coll. Cardiol 14, 1139–1148 (1989). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

