MiR-30a-5p and miR-153-3p regulate LPS-induced neuroinflammatory response and neuronal apoptosis by targeting NeuroD1
- PMID: 35651331
- PMCID: PMC9537026
- DOI: 10.5483/BMBRep.2022.55.9.061
MiR-30a-5p and miR-153-3p regulate LPS-induced neuroinflammatory response and neuronal apoptosis by targeting NeuroD1
Abstract
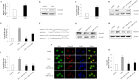
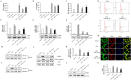
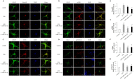
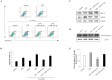
Neurogenic differentiation 1 (NeuroD1) is an essential transcription factor for neuronal differentiation, maturation, and survival, and is associated with inflammation in lipopolysaccharide (LPS)- induced glial cells; however, the concrete mechanisms are still ambiguous. Therefore, we investigated whether NeuroD1-targeting miRNAs affect inflammation and neuronal apoptosis, as well as the underlying mechanism. First, we confirmed that miR-30a-5p and miR-153-3p, which target NeuroD1, reduced NeuroD1 expression in microglia and astrocytes. In LPS-induced microglia, miR-30a-5p and miR-153-3p suppressed pro-inflammatory cytokines, reactive oxygen species, the phosphorylation of c-Jun N-terminal kinase, extracellular-signal-regulated kinase (ERK), and p38, and the expression of cyclooxygenase and inducible nitric oxide synthase (iNOS) via the NF-κB pathway. Moreover, miR-30a-5p and miR-153-3p inhibited the expression of NOD-like receptor pyrin domain containing 3 (NLRP3) inflammasomes, NLRP3, cleaved caspase-1, and IL-1β, which are involved in the innate immune response. In LPS-induced astrocytes, miR-30a-5p and miR-153-3p reduced ERK phosphorylation and iNOS expression via the STAT-3 pathway. Notably, miR-30a-5p exerted greater anti-inflammatory effects than miR-153-3p. Together, these results indicate that miR-30a-5p and miR-153-3p inhibit MAPK/NF-κB pathway in microglia as well as ERK/STAT-3 pathway in astrocytes to reduce LPS-induced neuronal apoptosis. This study highlights the importance of NeuroD1 in microglia and astrocytes neuroinflammation and suggests that it can be regulated by miR-30a-5p and miR-153-3p. [BMB Reports 2022; 55(9): 447-452].
Conflict of interest statement
The authors have no conflicting interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

