Potential of Polyethyleneimine as an Adjuvant To Prepare Long-Term and Potent Antifungal Nanovaccine
- PMID: 35651617
- PMCID: PMC9149211
- DOI: 10.3389/fimmu.2022.843684
Potential of Polyethyleneimine as an Adjuvant To Prepare Long-Term and Potent Antifungal Nanovaccine
Abstract
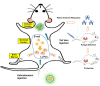
Background: Candida albicans infections are particularly prevalent in immunocompromised patients. Even with appropriate treatment with current antifungal drugs, the mortality rate of invasive candidiasis remains high. Many positive results have been achieved in the current vaccine development. There are also issues such as the vaccine's protective effect is not persistent. Considering the functionality and cost of the vaccine, it is important to develop safe and efficient new vaccines with long-term effects. In this paper, an antifungal nanovaccine with Polyethyleneimine (PEI) as adjuvant was constructed, which could elicit more effective and long-term immunity via stimulating B cells to differentiate into long-lived plasma cells.
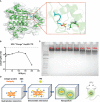
Materials and methods: Hsp90-CTD is an important target for protective antibodies during disseminated candidiasis. Hsp90-CTD was used as the antigen, then introduced SDS to "charge" the protein and added PEI to form the nanovaccine. Dynamic light scattering and transmission electron microscope were conducted to identify the size distribution, zeta potential, and morphology of nanovaccine. The antibody titers in mice immunized with the nanovaccine were measured by ELISA. The activation and maturation of long-lived plasma cells in bone marrow by nanovaccine were also investigated via flow cytometry. Finally, the kidney of mice infected with Candida albicans was stained with H&E and PAS to evaluate the protective effect of antibody in serum produced by immunized mice.
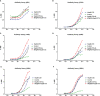
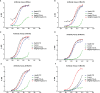
Results: Nanoparticles (NP) formed by Hsp90-CTD and PEI are small, uniform, and stable. NP had an average size of 116.2 nm with a PDI of 0.13. After immunizing mice with the nanovaccine, it was found that the nano-group produced antibodies faster and for a longer time. After 12 months of immunization, mice still had high and low levels of antibodies in their bodies. Results showed that the nanovaccine could promote the differentiation of B cells into long-lived plasma cells and maintain the long-term existence of antibodies in vivo. After immunization, the antibodies in mice could protect the mice infected by C. albicans.
Conclusion: As an adjuvant, PEI can promote the differentiation of B cells into long-lived plasma cells to maintain long-term antibodies in vivo. This strategy can be adapted for the future design of vaccines.
Keywords: fungal infections; long-lived plasma cell; long-term protection; nanoparticles; polyethylenimine.
Copyright © 2022 Jin, Dong, Liu, Liu, Qiu, Zhang, Zong, Hou, Guo, Sun, Chen, Dong, Li, An and Shen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Yang F, Xiao C, Qu J, Wang G. Structural Characterization of Low Molecular Weight Polysaccharide From Astragalus Membranaceus and its Immunologic Enhancement in Recombinant Protein Vaccine Against Systemic Candidiasis. Carbohydr Polym (2016) 145:48–55. doi: 10.1016/j.carbpol.2016.03.024 - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

