High-CBD Extract (CBD-X) Downregulates Cytokine Storm Systemically and Locally in Inflamed Lungs
- PMID: 35651623
- PMCID: PMC9149302
- DOI: 10.3389/fimmu.2022.875546
High-CBD Extract (CBD-X) Downregulates Cytokine Storm Systemically and Locally in Inflamed Lungs
Abstract
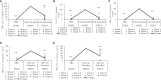
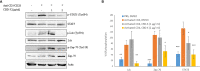
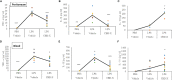
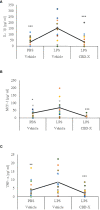
Cytokine storm refers to the dysregulated production of inflammatory mediators leading to hyperinflammation. They are often detrimental, and worsen the severity of COVID-19 and other infectious or inflammatory diseases. Cannabinoids are known to have anti-inflammatory effects but their possible therapeutic value on cytokine storms has not been fully elucidated. In vivo and ex vivo studies were carried out to investigate the effects of high-THC and high-CBD extracts on cytokine production in immune cells. Significant differences between the extracts were observed. Subsequent experiments focusing on a specific high CBD extract (CBD-X) showed significant reductions in pro-inflammatory cytokines in human-derived PBMCs, neutrophils and T cells. In vivo mouse studies, using a systemically inflamed mouse model, showed reductions in pro-inflammatory cytokines TNFα and IL-1β and a concurrent increase in the anti-inflammatory cytokine IL-10 in response to CBD-X extract treatment. Lung inflammation, as in severe COVID-19 disease, is characterized by increased T-cell homing to the lungs. Our investigation revealed that CBD-X extract impaired T-cell migration induced by the chemoattractant SDF1. In addition, the phosphorylation levels of T cell receptor (TCR) signaling proteins Lck and Zap70 were significantly reduced, demonstrating an inhibitory effect on the early events downstream to TCR activation. In a lung inflamed mouse model, we observed a reduction in leukocytes including neutrophil migration to the lungs and decreased levels of IL-1β, MCP-1, IL-6 and TNFα, in response to the administration of the high-CBD extract. The results presented in this work offer that certain high-CBD extract has a high potential in the management of pathological conditions, in which the secretion of cytokines is dysregulated, as it is in severe COVID-19 disease or other infectious or inflammatory diseases.
Keywords: CBD - cannabidiol; COVID - 19; cannabis; corona; cytokine storm and inflammation; lung inflammation.
Copyright © 2022 Aswad, Hamza, Pechkovsky, Zikrach, Popov, Zohar, Shahar and Louria-Hayon.
Conflict of interest statement
This study received funding from Raphael Pharmaceutical Inc. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or decision to submit it for publication. All authors declare no other competing interests.
Figures



Similar articles
-
Cannabinoids Alleviate the LPS-Induced Cytokine Storm via Attenuating NLRP3 Inflammasome Signaling and TYK2-Mediated STAT3 Signaling Pathways In Vitro.Cells. 2022 Apr 20;11(9):1391. doi: 10.3390/cells11091391. Cells. 2022. PMID: 35563697 Free PMC article.
-
Fighting the storm: could novel anti-TNFα and anti-IL-6 C. sativa cultivars tame cytokine storm in COVID-19?Aging (Albany NY). 2021 Jan 19;13(2):1571-1590. doi: 10.18632/aging.202500. Epub 2021 Jan 19. Aging (Albany NY). 2021. PMID: 33465050 Free PMC article.
-
Natural cannabinoids suppress the cytokine storm in sepsis-like in vitro model.Eur Cytokine Netw. 2020 Jun 1;31(2):50-58. doi: 10.1684/ecn.2020.0445. Eur Cytokine Netw. 2020. PMID: 32933892
-
The Effects of Cannabinoids on Pro- and Anti-Inflammatory Cytokines: A Systematic Review of In Vivo Studies.Cannabis Cannabinoid Res. 2021 Jun;6(3):177-195. doi: 10.1089/can.2020.0105. Epub 2021 Apr 28. Cannabis Cannabinoid Res. 2021. PMID: 33998900 Free PMC article.
-
Bioactive Molecules from Tropical American Plants: Potential Anti-Inflammatory Agents for Cytokine Storm Management.Molecules. 2025 Mar 27;30(7):1486. doi: 10.3390/molecules30071486. Molecules. 2025. PMID: 40286093 Free PMC article. Review.
Cited by
-
High-CBD Extract (CBD-X) in Asthma Management: Reducing Th2-Driven Cytokine Secretion and Neutrophil/Eosinophil Activity.Pharmaceuticals (Basel). 2024 Oct 17;17(10):1382. doi: 10.3390/ph17101382. Pharmaceuticals (Basel). 2024. PMID: 39459021 Free PMC article.
-
Antiviral activities of hemp cannabinoids.Clin Sci (Lond). 2023 Apr 26;137(8):633-643. doi: 10.1042/CS20220193. Clin Sci (Lond). 2023. PMID: 37083031 Free PMC article.
-
Cannabis and Rheumatoid Arthritis: A Scoping Review Evaluating the Benefits, Risks, and Future Research Directions.Rambam Maimonides Med J. 2023 Oct 29;14(4):e0022. doi: 10.5041/RMMJ.10509. Rambam Maimonides Med J. 2023. PMID: 37917863 Free PMC article.
-
Differential Effects of D9 Tetrahydrocannabinol (THC)- and Cannabidiol (CBD)-Based Cannabinoid Treatments on Macrophage Immune Function In Vitro and on Gastrointestinal Inflammation in a Murine Model.Biomedicines. 2022 Jul 26;10(8):1793. doi: 10.3390/biomedicines10081793. Biomedicines. 2022. PMID: 35892693 Free PMC article.
-
Encapsulation of Hemp (Cannabis sativa L.) Essential Oils into Nanoemulsions for Potential Therapeutic Applications: Assessment of Cytotoxicological Profiles.Molecules. 2023 Sep 7;28(18):6479. doi: 10.3390/molecules28186479. Molecules. 2023. PMID: 37764255 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

