Efficacy of Isatuximab With Pomalidomide and Dexamethasone in Relapsed Myeloma: Results of a UK-Wide Real-World Dataset
- PMID: 35651713
- PMCID: PMC9148694
- DOI: 10.1097/HS9.0000000000000738
Efficacy of Isatuximab With Pomalidomide and Dexamethasone in Relapsed Myeloma: Results of a UK-Wide Real-World Dataset
Abstract
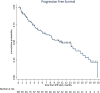
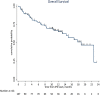
Real-world data on the efficacy and tolerability of isatuximab with pomalidomide and dexamethasone (IsaPomDex) in relapsed/refractory myeloma patients have not been reported. In this UK-wide retrospective study, IsaPomDex outcomes were evaluated across 24 routine care cancer centers. The primary endpoint was overall response rate (ORR). Secondary endpoints included progression-free survival (PFS), duration of response (DOR) for patients who achieved an objective response (≥partial response [PR]), and adverse events (AEs). In a total cohort 107 patients, median follow up (interquartile range [IQR]) was 12.1 months (10.1-18.6 mo), median age (IQR) was 69 years (61-77). Median (IQR) Charlson Comorbidity Index (CCI) score was 3 (2-4); 43% had eGFR <60 mL/min. Median (IQR) number of prior therapies was 3 (3-3). Median (IQR) number of IsaPomDex cycles administered was 7 (3-13). ORR was 66.4%, with responses categorized as ≥ very good partial response: 31.8%, PR: 34.6%, stable disease: 15.9%, progressive disease: 15%, and unknown 2.8%. Median PFS was 10.9 months. Median DOR was 10.3 months. There was no statistical difference in median PFS by age (<65: 10.2 versus 65-74 13.2 versus ≥75: 8.5 mo, log-rank P = 0.4157), by CCI score (<4: 10.2 mo versus ≥4: 13.2, log-rank P = 0.6531), but inferior PFS was observed with renal impairment (≥60: 13.2 versus <60: 7.9 mo, log-rank P = 0.0408). Median OS was 18.8 months. After a median of 4 cycles, any grade AEs were experienced by 87.9% of patients. The most common ≥G3 AEs were neutropenia (45.8%), infections (18.7%), and thrombocytopenia (14%). Our UK-wide IsaPomDex study demonstrated encouraging efficacy outcomes in the real world, comparable to ICARIA-MM trial.
Copyright © 2022 the Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the European Hematology Association.
Figures


References
-
- Lonial S, Weiss BM, Usmani SZ, et al. . Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet. 2016;387:1551–1560. - PubMed
-
- Richardson PG, Beksaç M, Špička I, et al. . Isatuximab for the treatment of relapsed/refractory multiple myeloma. Expert Opin Biol Ther. 2020;20:1395–1404. - PubMed
-
- National Institute for Health and Care Excellence. Isatuximab with pomalidomide and dexamethasone for treating relapsed and refractory multiple myeloma. 2020. Available at: https://www.nice.org.uk/guidance/TA658. Accessed June 22, 2021.
-
- Attal M, Richardson PG, Rajkumar SV, et al. . Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): a randomised, multicentre, open-label, phase 3 study. Lancet. 2019;394:2096–2107. - PubMed
-
- Zweegman S, Engelhardt M, Larocca A. Elderly patients with multiple myeloma: towards a frailty approach? Curr Opin Oncol. 2017;29:315–321. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
