Validation of Effective Extracellular Vesicles Isolation Methods Adapted to Field Studies in Malaria Endemic Regions
- PMID: 35652104
- PMCID: PMC9149222
- DOI: 10.3389/fcell.2022.812244
Validation of Effective Extracellular Vesicles Isolation Methods Adapted to Field Studies in Malaria Endemic Regions
Abstract
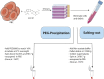
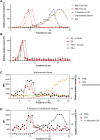
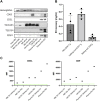
Malaria affects the poorer regions of the world and is of tremendous health and economic burden for developing countries. Extracellular vesicles (EVs) are small vesicles released by almost any cells in the human body, including malaria infected red blood cells. Recent evidence shows that EVs might contribute to the pathogenesis of malaria. In addition, EVs hold considerable value in biomarker discovery. However, there are still significant gaps in our understanding of EV biology. So far most of our knowledge about EVs in malaria comes from in vitro work. More field studies are required to gain insight into their contribution to the disease and pathogenesis under physiological conditions. However, to perform research on EVs in low-income regions might be challenging due to the lack of appropriate equipment to isolate EVs. Therefore, there is a need to develop and validate EV extraction protocols applicable to poorly equipped laboratories. We established and validated two protocols for EV isolation from cell culture supernatants, rodent and human plasma. We compared polyethylene glycol (PEG) and salting out (SA) with sodium acetate for precipitation of EVs. We then characterized the EVs by Transmission Electron Microscopy (TEM), Western Blot, Size-exclusion chromatography (SEC), bead-based flow cytometry and protein quantification. Both protocols resulted in efficient purification of EVs without the need of expensive material or ultracentrifugation. Furthermore, the procedure is easily scalable to work with large and small sample volumes. Here, we propose that both of our approaches can be used in resource limited countries, therefore further helping to close the gap in knowledge of EVs during malaria.
Keywords: Plasmodium falciparum; extracellular vesicles; malaria; microvesicles; polyethylene glycol precipitation; salting-out.
Copyright © 2022 Zoia, Yesodha Subramanian, Eriksson, Ravi, Yaghmaei, Fellay, Scolari, Walch and Mantel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Antwi-Baffour S., Malibha-Pinchbeck M., Stratton D., Jorfi S., Lange S., Inal J. (2020). Plasma mEV Levels in Ghanain Malaria Patients with Low Parasitaemia Are Higher Than Those of Healthy Controls, Raising the Potential for Parasite Markers in mEVs as Diagnostic Targets. J. Extracell Vesicles 9, 1697124. 10.1080/20013078.2019.1697124 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

