Emerging insights into synapse dysregulation in Alzheimer's disease
- PMID: 35652120
- PMCID: PMC9149787
- DOI: 10.1093/braincomms/fcac083
Emerging insights into synapse dysregulation in Alzheimer's disease
Abstract
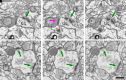
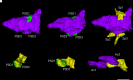
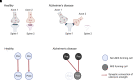
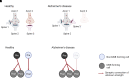
Alzheimer's disease is the leading cause of dementia and a growing worldwide problem, with its incidence expected to increase in the coming years. Since synapse loss is a major pathology and is correlated with symptoms in Alzheimer's disease, synapse dysfunction and loss may underlie pathophysiology. In this context, this review focuses on emerging insights into synaptic changes at the ultrastructural level. The three-dimensional electron microscopy technique unequivocally detects all types of synapses, including multi-synapses, which are indicators of synaptic connectivity between neurons. In recent years it has become feasible to perform sophisticated three-dimensional electron microscopy analyses on post-mortem human Alzheimer's disease brain as tissue preservation and electron microscopy techniques have improved. This ultrastructural analysis found that synapse loss does not always precede neuronal loss, as long believed. For instance, in the transentorhinal cortex and area CA1 of the hippocampus, synapse loss does not precede neuronal loss. However, in the entorhinal cortex, synapse loss precedes neuronal loss. Moreover, the ultrastructural analysis provides details about synapse morphology. For example, changes in excitatory synapses' post-synaptic densities, with fragmented postsynaptic densities increasing at the expense of perforated synapses, are seen in Alzheimer's disease brain. Further, multi-synapses also appear to be altered in Alzheimer's disease by doubling the abundance of multi-innervated spines in the transentorhinal cortex of Alzheimer's disease brain. Collectively, these recent ultrastructural analyses highlight distinct synaptic phenotypes in different Alzheimer's disease brain regions and broaden the understanding of synapse alterations, which may unravel some new therapeutic targets.
Keywords: Alzheimer’s disease; multi-innervated spine; multi-spine bouton; synapses; three-dimensional electron microscopy.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures


References
-
- DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: Correlation with cognitive severity. Ann Neurol 1990;27(5):457–464. - PubMed
-
- Scheff SW, DeKosky ST, Price DA. Quantitative assessment of cortical synaptic density in Alzheimer’s disease. Neurobiol Aging 1990;11(1):29–37. - PubMed
-
- DeKosky ST, Scheff SW, Styren SD. Structural correlates of cognition in dementia: Quantification and assessment of synapse change. Neurodegeneration 1996;5(4):417–421. - PubMed
-
- Terry RD, Masliah E, Salmon DP, et al. . Physical basis of cognitive alterations in alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Ann Neurol 1991;30(4):572–580. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
