Quantitative magnetization transfer imaging in relapsing-remitting multiple sclerosis: a systematic review and meta-analysis
- PMID: 35652121
- PMCID: PMC9149789
- DOI: 10.1093/braincomms/fcac088
Quantitative magnetization transfer imaging in relapsing-remitting multiple sclerosis: a systematic review and meta-analysis
Abstract
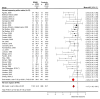
Myelin-sensitive MRI such as magnetization transfer imaging has been widely used in multiple sclerosis. The influence of methodology and differences in disease subtype on imaging findings is, however, not well established. Here, we systematically review magnetization transfer brain imaging findings in relapsing-remitting multiple sclerosis. We examine how methodological differences, disease effects and their interaction influence magnetization transfer imaging measures. Articles published before 06/01/2021 were retrieved from online databases (PubMed, EMBASE and Web of Science) with search terms including 'magnetization transfer' and 'brain' for systematic review, according to a pre-defined protocol. Only studies that used human in vivo quantitative magnetization transfer imaging in adults with relapsing-remitting multiple sclerosis (with or without healthy controls) were included. Additional data from relapsing-remitting multiple sclerosis subjects acquired in other studies comprising mixed disease subtypes were included in meta-analyses. Data including sample size, MRI acquisition protocol parameters, treatments and clinical findings were extracted and qualitatively synthesized. Where possible, effect sizes were calculated for meta-analyses to determine magnetization transfer (i) differences between patients and healthy controls; (ii) longitudinal change and (iii) relationships with clinical disability in relapsing-remitting multiple sclerosis. Eighty-six studies met inclusion criteria. MRI acquisition parameters varied widely, and were also underreported. The majority of studies examined the magnetization transfer ratio in white matter, but magnetization transfer metrics, brain regions examined and results were heterogeneous. The analysis demonstrated a risk of bias due to selective reporting and small sample sizes. The pooled random-effects meta-analysis across all brain compartments revealed magnetization transfer ratio was 1.17 per cent units (95% CI -1.42 to -0.91) lower in relapsing-remitting multiple sclerosis than healthy controls (z-value: -8.99, P < 0.001, 46 studies). Linear mixed-model analysis did not show a significant longitudinal change in magnetization transfer ratio across all brain regions [β = 0.12 (-0.56 to 0.80), t-value = 0.35, P = 0.724, 14 studies] or normal-appearing white matter alone [β = 0.037 (-0.14 to 0.22), t-value = 0.41, P = 0.68, eight studies]. There was a significant negative association between the magnetization transfer ratio and clinical disability, as assessed by the Expanded Disability Status Scale [r = -0.32 (95% CI -0.46 to -0.17); z-value = -4.33, P < 0.001, 13 studies]. Evidence suggests that magnetization transfer imaging metrics are sensitive to pathological brain changes in relapsing-remitting multiple sclerosis, although effect sizes were small in comparison to inter-study variability. Recommendations include: better harmonized magnetization transfer acquisition protocols with detailed methodological reporting standards; larger, well-phenotyped cohorts, including healthy controls; and, further exploration of techniques such as magnetization transfer saturation or inhomogeneous magnetization transfer ratio.
Keywords: brain; magnetization transfer; multiple sclerosis; relapsing-remitting; systematic review.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures





References
-
- National Multiple Sclerosis Society . Accessed 21 June 2019. https://www.nationalmssociety.org/What-is-MS/Definition-of-MS.
-
- Barkhof F. MRI in multiple sclerosis: Correlation with expanded disability status scale (EDSS). Mult Scler. 1999;5(4):283–286. - PubMed
-
- Li DK, Held U, Petkau J, et al. . MRI T2 lesion burden in multiple sclerosis: A plateauing relationship with clinical disability. Neurology. 2006;66(9):1384–1389. - PubMed
-
- Agosta F, Rovaris M, Pagani E, Sormani MP, Comi G, Filippi M. Magnetization transfer MRI metrics predict the accumulation of disability 8 years later in patients with multiple sclerosis. Brain. 2006;129(Pt 10):2620–2627. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
