Magnesium-Encapsulated Injectable Hydrogel and 3D-Engineered Polycaprolactone Conduit Facilitate Peripheral Nerve Regeneration
- PMID: 35652188
- PMCID: PMC9313484
- DOI: 10.1002/advs.202202102
Magnesium-Encapsulated Injectable Hydrogel and 3D-Engineered Polycaprolactone Conduit Facilitate Peripheral Nerve Regeneration
Abstract
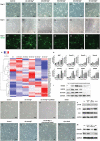
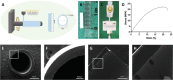
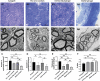
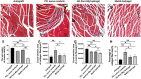
Peripheral nerve injury is a challenging orthopedic condition that can be treated by autograft transplantation, a gold standard treatment in the current clinical setting. Nevertheless, limited availability of autografts and potential morbidities in donors hampers its widespread application. Bioactive scaffold-based tissue engineering is a promising strategy to promote nerve regeneration. Additionally, magnesium (Mg) ions enhance nerve regeneration; however, an effectively controlled delivery vehicle is necessary to optimize their in vivo therapeutic effects. Herein, a bisphosphonate-based injectable hydrogel exhibiting sustained Mg2+ delivery for peripheral nerve regeneration is developed. It is observed that Mg2+ promoted neurite outgrowth in a concentration-dependent manner by activating the PI3K/Akt signaling pathway and Sema5b. Moreover, implantation of polycaprolactone (PCL) conduits filled with Mg2+ -releasing hydrogel in 10 mm nerve defects in rats significantly enhanced axon regeneration and remyelination at 12 weeks post-operation compared to the controls (blank conduits or conduits filled with Mg2+ -absent hydrogel). Functional recovery analysis reveals enhanced reinnervation in the animals treated with the Mg2+ -releasing hydrogel compared to that in the control groups. In summary, the Mg2+ -releasing hydrogel combined with the 3D-engineered PCL conduit promotes peripheral nerve regeneration and functional recovery. Thus, a new strategy to facilitate the repair of challenging peripheral nerve injuries is proposed.
Keywords: hydrogel; magnesium; peripheral nerve regeneration.
© 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
