Coproporphyrin I as an Endogenous Biomarker to Detect Reduced OATP1B Activity and Shift in Elimination Route in Chronic Kidney Disease
- PMID: 35652251
- PMCID: PMC9540787
- DOI: 10.1002/cpt.2672
Coproporphyrin I as an Endogenous Biomarker to Detect Reduced OATP1B Activity and Shift in Elimination Route in Chronic Kidney Disease
Abstract
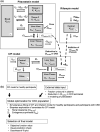
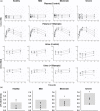
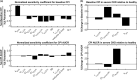
Coproporphyrin I (CPI) is an endogenous biomarker of organic anion transporting polypeptide 1B transporter (OATP1B). CPI plasma baseline was reported to increase with severity of chronic kidney disease (CKD). Further, ratio of CPI area under the plasma concentration-time curve (AUCR) in the presence/absence of OATP1B inhibitor rifampin was higher in patients with CKD compared with healthy participants, in contrast to pitavastatin (a clinical OATP1B probe). This study investigated mechanism(s) contributing to altered CPI baseline in patients with CKD by extending a previously developed physiologically-based pharmacokinetic (PBPK) model to this patient population. CKD-related covariates were evaluated in a stepwise manner on CPI fraction unbound in plasma (fu,p ), OATP1B-mediated hepatic uptake clearance (CLactive ), renal clearance (CLR ), and endogenous synthesis (ksyn ). The CPI model successfully recovered increased baseline and rifampin-mediated AUCR in patients with CKD by accounting for the following disease-related changes: 13% increase in fu,p , 29% and 39% decrease in CLactive in mild and moderate to severe CKD, respectively, decrease in CLR proportional to decline in glomerular filtration rate, and 27% decrease in ksyn in severe CKD. Almost complete decline in CPI renal elimination in severe CKD increased its fraction transported by OATP1B, rationalizing differences in the CPI-rifampin interaction observed between healthy participants and patients with CKD. In conclusion, mechanistic modeling performed here supports CKD-related decrease in OATP1B function to inform prospective PBPK modeling of OATP1B-mediated drug-drug interaction in these patients. Monitoring of CPI allows detection of CKD-drug interaction risk for OATP1B drugs with combined hepatic and renal elimination which may be underestimated by extrapolating the interaction risk based on pitavastatin data in healthy participants.
© 2022 The Authors. Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The other authors declared no competing interests for this work.
Figures


References
-
- Miners, J.O. , Yang, X. , Knights, K.M. & Zhang, L. The role of the kidney in drug elimination: transport, metabolism, and the impact of kidney disease on drug clearance. Clin. Pharmacol. Ther. 102, 436–449 (2017). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

