Bioengineering trends in female reproduction: a systematic review
- PMID: 35652272
- PMCID: PMC9629485
- DOI: 10.1093/humupd/dmac025
Bioengineering trends in female reproduction: a systematic review
Abstract
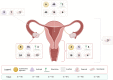
Background: To provide the optimal milieu for implantation and fetal development, the female reproductive system must orchestrate uterine dynamics with the appropriate hormones produced by the ovaries. Mature oocytes may be fertilized in the fallopian tubes, and the resulting zygote is transported toward the uterus, where it can implant and continue developing. The cervix acts as a physical barrier to protect the fetus throughout pregnancy, and the vagina acts as a birth canal (involving uterine and cervix mechanisms) and facilitates copulation. Fertility can be compromised by pathologies that affect any of these organs or processes, and therefore, being able to accurately model them or restore their function is of paramount importance in applied and translational research. However, innate differences in human and animal model reproductive tracts, and the static nature of 2D cell/tissue culture techniques, necessitate continued research and development of dynamic and more complex in vitro platforms, ex vivo approaches and in vivo therapies to study and support reproductive biology. To meet this need, bioengineering is propelling the research on female reproduction into a new dimension through a wide range of potential applications and preclinical models, and the burgeoning number and variety of studies makes for a rapidly changing state of the field.
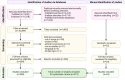
Objective and rationale: This review aims to summarize the mounting evidence on bioengineering strategies, platforms and therapies currently available and under development in the context of female reproductive medicine, in order to further understand female reproductive biology and provide new options for fertility restoration. Specifically, techniques used in, or for, the uterus (endometrium and myometrium), ovary, fallopian tubes, cervix and vagina will be discussed.
Search methods: A systematic search of full-text articles available in PubMed and Embase databases was conducted to identify relevant studies published between January 2000 and September 2021. The search terms included: bioengineering, reproduction, artificial, biomaterial, microfluidic, bioprinting, organoid, hydrogel, scaffold, uterus, endometrium, ovary, fallopian tubes, oviduct, cervix, vagina, endometriosis, adenomyosis, uterine fibroids, chlamydia, Asherman's syndrome, intrauterine adhesions, uterine polyps, polycystic ovary syndrome and primary ovarian insufficiency. Additional studies were identified by manually searching the references of the selected articles and of complementary reviews. Eligibility criteria included original, rigorous and accessible peer-reviewed work, published in English, on female reproductive bioengineering techniques in preclinical (in vitro/in vivo/ex vivo) and/or clinical testing phases.
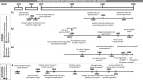
Outcomes: Out of the 10 390 records identified, 312 studies were included for systematic review. Owing to inconsistencies in the study measurements and designs, the findings were assessed qualitatively rather than by meta-analysis. Hydrogels and scaffolds were commonly applied in various bioengineering-related studies of the female reproductive tract. Emerging technologies, such as organoids and bioprinting, offered personalized diagnoses and alternative treatment options, respectively. Promising microfluidic systems combining various bioengineering approaches have also shown translational value.
Wider implications: The complexity of the molecular, endocrine and tissue-level interactions regulating female reproduction present challenges for bioengineering approaches to replace female reproductive organs. However, interdisciplinary work is providing valuable insight into the physicochemical properties necessary for reproductive biological processes to occur. Defining the landscape of reproductive bioengineering technologies currently available and under development for women can provide alternative models for toxicology/drug testing, ex vivo fertility options, clinical therapies and a basis for future organ regeneration studies.
Keywords: bioengineering; cervix; endometrium; fallopian tubes; female reproduction; fertility restoration; myometrium; ovary; uterus; vagina.
© The Author(s) 2022. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Figures