Methodological survey of missing outcome data in an alteplase for ischemic stroke meta-analysis
- PMID: 35652287
- PMCID: PMC9541760
- DOI: 10.1111/ane.13656
Methodological survey of missing outcome data in an alteplase for ischemic stroke meta-analysis
Abstract
Objective: Recent national guidelines recommend alteplase treatment for ischemic stroke within 4.5 h of symptom-onset based on meta-analyses of randomized controlled clinical trials (RCT). A detailed description of missing outcome data (MOD) due to participant loss to follow-up has never been published. The objective of this study was to perform a methodlogical survey on missing outcome data in an alteplase for ischemic stroke meta-analysis.
Materials and methods: A methodological survey was performed on a chosen meta-analysis of alteplase for ischemic stroke RCTs that most closely aligns with recent national guideline recommendations. Data were collected to assess the number of participants lost to follow-up; differential lost to follow-up between allocation groups; baseline characteristics of those lost to follow-up; and the imputation methods used by individual trials and the chosen meta-analysis. The number of participants lost to follow-up was compared with the fragility index; and repeated for individually positive RCTs in the meta-analysis.
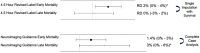
Results: The methodological survey revealed a substantial degree of missing information regarding MOD in the chosen meta-analysis and in individual RCTs. Single imputation was exclusively used in all RCTs and in the meta-analysis. The number of participants lost to follow-up was greater than the fragility index in the chosen meta-analysis and individually positive component RCTs suggesting that MOD may impact the direction of the reported effect or effect size.
Conclusion: This methodological survey of an alteplase for ischemic stroke meta-analysis revealed MOD may be an important source of unrecognized bias. This survey highlights the need for sensitivity analyses using more robust methods of imputation.
Keywords: alteplase; bias; ischemic stroke; meta-analysis; missing outcome data.
© 2022 The Author. Acta Neurologica Scandinavica published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures
Similar articles
-
A methodological assessment of randomization integrity in alteplase for acute ischemic stroke individual patient data meta-analyses.PLoS One. 2025 Mar 19;20(3):e0315342. doi: 10.1371/journal.pone.0315342. eCollection 2025. PLoS One. 2025. PMID: 40106441 Free PMC article.
-
In ischemic stroke, IV alteplase within 3 h of onset improves functional outcome vs. control.Ann Intern Med. 2022 Sep;175(9):JC107. doi: 10.7326/J22-0069. Epub 2022 Sep 6. Ann Intern Med. 2022. PMID: 36063553
-
Different doses of tenecteplase vs alteplase in thrombolysis therapy of acute ischemic stroke: evidence from randomized controlled trials.Drug Des Devel Ther. 2018 Jul 6;12:2071-2084. doi: 10.2147/DDDT.S170803. eCollection 2018. Drug Des Devel Ther. 2018. PMID: 30013325 Free PMC article. Clinical Trial.
-
Intravenous alteplase for stroke with unknown time of onset guided by advanced imaging: systematic review and meta-analysis of individual patient data.Lancet. 2020 Nov 14;396(10262):1574-1584. doi: 10.1016/S0140-6736(20)32163-2. Epub 2020 Nov 8. Lancet. 2020. PMID: 33176180 Free PMC article.
-
Alteplase and ischaemic stroke: have new reviews of old data helped?Lancet Neurol. 2005 Apr;4(4):249-53. doi: 10.1016/S1474-4422(05)70044-2. Lancet Neurol. 2005. PMID: 15778104 Review.
Cited by
-
A methodological assessment of randomization integrity in alteplase for acute ischemic stroke individual patient data meta-analyses.PLoS One. 2025 Mar 19;20(3):e0315342. doi: 10.1371/journal.pone.0315342. eCollection 2025. PLoS One. 2025. PMID: 40106441 Free PMC article.
-
Real world data in mechanical thrombectomy: who are we losing to follow-up?J Neurointerv Surg. 2024 Apr 23;16(5):471-477. doi: 10.1136/jnis-2023-020435. J Neurointerv Surg. 2024. PMID: 37460214 Free PMC article.
References
-
- Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 update to the 2018 guidelines for the early Management of Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344‐e418. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

