Skeletal Stem/Progenitor Cells in Periosteum and Skeletal Muscle Share a Common Molecular Response to Bone Injury
- PMID: 35652423
- PMCID: PMC9543664
- DOI: 10.1002/jbmr.4616
Skeletal Stem/Progenitor Cells in Periosteum and Skeletal Muscle Share a Common Molecular Response to Bone Injury
Abstract
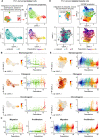
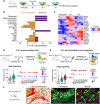
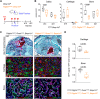
Bone regeneration involves skeletal stem/progenitor cells (SSPCs) recruited from bone marrow, periosteum, and adjacent skeletal muscle. To achieve bone reconstitution after injury, a coordinated cellular and molecular response is required from these cell populations. Here, we show that SSPCs from periosteum and skeletal muscle are enriched in osteochondral progenitors, and more efficiently contribute to endochondral ossification during fracture repair as compared to bone-marrow stromal cells. Single-cell RNA sequencing (RNAseq) analyses of periosteal cells reveal the cellular heterogeneity of periosteum at steady state and in response to bone fracture. Upon fracture, both periosteal and skeletal muscle SSPCs transition from a stem/progenitor to a fibrogenic state prior to chondrogenesis. This common activation pattern in periosteum and skeletal muscle SSPCs is mediated by bone morphogenetic protein (BMP) signaling. Functionally, Bmpr1a gene inactivation in platelet-derived growth factor receptor alpha (Pdgfra)-derived SSPCs impairs bone healing and decreases SSPC proliferation, migration, and osteochondral differentiation. These results uncover a coordinated molecular program driving SSPC activation in periosteum and skeletal muscle toward endochondral ossification during bone regeneration. © 2022 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).
Keywords: BMP PATHWAY; BONE REPAIR; PERIOSTEUM; SINGLE CELL RNA SEQUENCING; SKELETAL STEM/PROGENITOR CELLS.
© 2022 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).
Conflict of interest statement
Authors declare no competing interests.
Figures




References
-
- Roberts SJ, van Gastel N, Carmeliet G, Luyten FP. Uncovering the periosteum for skeletal regeneration: the stem cell that lies beneath. Bone. 2015;70:10‐18. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

