Dynamically Responsive Scaffolds from Microfluidic 3D Printing for Skin Flap Regeneration
- PMID: 35652496
- PMCID: PMC9353450
- DOI: 10.1002/advs.202201155
Dynamically Responsive Scaffolds from Microfluidic 3D Printing for Skin Flap Regeneration
Abstract
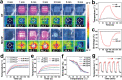
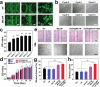
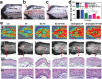
Biological scaffolds hold promising perspectives for random skin flap regeneration, while the practical application is greatly limited by their insufficient vascularization ability and the lack of responsiveness during the dynamical healing process. Herein, a novel MXene-incorporated hollow fibrous (MX-HF) scaffold with dynamically responsive channels is presented for promoting vascularization and skin flap regeneration by using a microfluidic-assisted 3D printing strategy. Benefiting from the photothermal conversion capacity of the MXene nanosheets and temperature-responsive ability of poly(NIPAM) hydrogels in the MX-HF scaffolds, they display a near-infrared (NIR)-responsive shrinkage/swelling behavior, which facilitates the cell penetration into the scaffold channels from the surrounding environment. Moreover, by incorporating vascular endothelial growth factor (VEGF) into the hydrogel matrix for controllable delivery, the MX-HF scaffolds can achieve promoted proliferation, migration, and proangiogenic effects of endothelial cells under NIR irradiation. It is further demonstrated in vivo that the NIR-responsive VEGF@MX-HF scaffolds can effectively improve skin flap survival by promoting angiogenesis, decreasing inflammation, and attenuating apoptosis in skin flaps. Thus, it is believed that such responsive MX-HF scaffolds are promising candidates for clinical random skin flap regeneration as well as other diverse tissue engineering applications.
Keywords: 3D printing; microfluidics; photothermal; regeneration; scaffold; vascularization.
© 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- a) Marks H., Bucknor A., Roussakis E., Nowell N., Kamali P., Cascales J. P., Kazei D., Lin S. J., Evans C. L., Sci. Adv. 2020, 6, eabd1061; - PMC - PubMed
- b) Khan Aadil A., Paget James T., McLaughlin M., Kyula Joan N., Wilkinson Michelle J., Pencavel T., Mansfield D., Roulstone V., Seth R., Halle M., Somaiah N., Boult Jessica K. R., Robinson Simon P., Pandha Hardev S., Vile Richard G., Melcher Alan A., Harris Paul A., Harrington Kevin J., Sci. Transl. Med. 2018, 10, eaar2041. - PMC - PubMed
-
- Sun X., Lang Q., Zhang H., Cheng L., Zhang Y., Pan G., Zhao X., Yang H., Zhang Y., Santos H. A., Cui W., Adv. Funct. Mater. 2017, 27, 1604617.
-
- a) Cai Z., Saiding Q., Cheng L., Zhang L., Wang Z., Wang F., Chen X., Chen G., Deng L., Cui W., Bioactive Mater. 2021, 6, 4506; - PMC - PubMed
- b) Zhang L., Chen L., Xiang Y., Liu Z., Mao X., Zhang L., Deng L., Zhang Y., Cheng L., Sun X., Cui W., Chem. Eng. J. 2021, 406, 126839;
- c) Zhang L., Xiang Y., Zhang H., Cheng L., Mao X., An N., Zhang L., Zhou J., Deng L., Zhang Y., Sun X., Santos H. A., Cui W., Adv. Sci. 2020, 7, 1903553; - PMC - PubMed
- d) Mao X., Liu L., Cheng L., Cheng R., Zhang L., Deng L., Sun X., Zhang Y., Sarmento B., Cui W., J. Controlled Release 2019, 297, 91. - PubMed
-
- a) Cui C., Sun S., Wu S., Chen S., Ma J., Zhou F., Eng. Regener. 2021, 2, 82;
- b) Li X., Cho B., Martin R., Seu M., Zhang C., Zhou Z., Choi Ji S., Jiang X., Chen L., Walia G., Yan J., Callanan M., Liu H., Colbert K., Morrissette‐McAlmon J., Grayson W., Reddy S., Sacks Justin M., Mao H.‐Q., Sci. Transl. Med. 2019, 11, eaau6210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
