Isothiourea-Catalyzed Enantioselective Michael Addition of Malonates to α,β-Unsaturated Aryl Esters
- PMID: 35652512
- PMCID: PMC9278409
- DOI: 10.1021/acs.orglett.2c01486
Isothiourea-Catalyzed Enantioselective Michael Addition of Malonates to α,β-Unsaturated Aryl Esters
Abstract
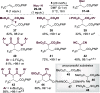
An enantioselective Michael addition of malonates to α,β-unsaturated para-nitrophenyl esters was achieved using the Lewis basic isothiourea HyperBTM, giving excellent levels of product enantioselectivity (up to >99:1 enantiomeric ratio) in good yields and with complete regioselectivity (>20:1 regioselectivity ratio) in the presence of alternative (phenyl ketone and ethyl ester) Michael acceptors. Density functional theory calculations indicate that N-acylation is rate-limiting. This constitutes a rare example of a highly enantioselective addition of simple, readily available malonates to α,β-unsaturated esters.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Isothiourea-catalyzed formal enantioselective conjugate addition of benzophenone imines to β-fluorinated α,β-unsaturated esters.Chem Commun (Camb). 2022 Jun 16;58(49):6886-6889. doi: 10.1039/d2cc01936a. Chem Commun (Camb). 2022. PMID: 35635248
-
Aryloxide-Facilitated Catalyst Turnover in Enantioselective α,β-Unsaturated Acyl Ammonium Catalysis.Angew Chem Int Ed Engl. 2017 Sep 25;56(40):12282-12287. doi: 10.1002/anie.201706402. Epub 2017 Aug 25. Angew Chem Int Ed Engl. 2017. PMID: 28791763 Free PMC article.
-
Isothiourea-Catalyzed Enantioselective Synthesis of Tetrahydro-α-carbolinones.Org Lett. 2020 Feb 21;22(4):1301-1305. doi: 10.1021/acs.orglett.9b04615. Epub 2020 Feb 6. Org Lett. 2020. PMID: 32027139
-
Base-free Enantioselective C(1)-Ammonium Enolate Catalysis Exploiting Aryloxides: A Synthetic and Mechanistic Study.Angew Chem Int Ed Engl. 2019 Oct 14;58(42):15111-15119. doi: 10.1002/anie.201908627. Epub 2019 Sep 12. Angew Chem Int Ed Engl. 2019. PMID: 31436380 Review.
-
Enantioselective total syntheses of several bioactive natural products based on the development of practical asymmetric catalysis.Chem Pharm Bull (Tokyo). 2004 Sep;52(9):1031-52. doi: 10.1248/cpb.52.1031. Chem Pharm Bull (Tokyo). 2004. PMID: 15340187 Review.
Cited by
-
Enantioselective isothiourea-catalysed reversible Michael addition of aryl esters to 2-benzylidene malononitriles.Chem Sci. 2023 Jun 2;14(27):7537-7544. doi: 10.1039/d3sc02101g. eCollection 2023 Jul 12. Chem Sci. 2023. PMID: 37449062 Free PMC article.
-
Stereoselective Processes Based on σ-Hole Interactions.Molecules. 2022 Jul 20;27(14):4625. doi: 10.3390/molecules27144625. Molecules. 2022. PMID: 35889497 Free PMC article. Review.
-
Understanding divergent substrate stereoselectivity in the isothiourea-catalysed conjugate addition of cyclic α-substituted β-ketoesters to α,β-unsaturated aryl esters.Chem Sci. 2023 Nov 21;14(48):14146-14156. doi: 10.1039/d3sc05470e. eCollection 2023 Dec 13. Chem Sci. 2023. PMID: 38098722 Free PMC article.
-
Isothiourea catalysed enantioselective generation of point and axially chiral iminothia- and iminoselenazinanones.Chem Sci. 2025 Apr 30;16(23):10494-10502. doi: 10.1039/d5sc02435h. eCollection 2025 Jun 11. Chem Sci. 2025. PMID: 40365056 Free PMC article.
-
Recent Advances in the Synthesis of 3,4-Dihydropyran-2-Ones Organocatalyzed by N-Heterocyclic Carbenes.Molecules. 2023 Apr 26;28(9):3743. doi: 10.3390/molecules28093743. Molecules. 2023. PMID: 37175154 Free PMC article. Review.
References
-
-
For recent reviews on the enantioselective organocatalytic Michael reaction, see
- Parella R.; Jakkampudi S.; Zhao J. C.-G. Recent Applications of Asymmetric Organocatalytic Methods in Total Synthesis. ChemistrySelect 2021, 6, 2252–2280. 10.1002/slct.202004196. - DOI
- Scheffler U.; Mahrwald R. Recent Advances in Organocatalytic Methods for Asymmetric C–C Bond Formation. Chem.—Eur. J. 2013, 19, 14346–14396. 10.1002/chem.201301996. - DOI - PubMed
- Zhang Y.; Wang W. Recent Advances in Organocatalytic Asymmetric Michael Reactions. Catal. Sci. Technol. 2012, 2, 42–53. 10.1039/C1CY00334H. - DOI
-
-
- Takaya Y.; Senda T.; Kurushima H.; Ogasawara M.; Hayashi T. Rhodium-catalyzed Asymmetric 1,4-Addition of Arylboron Reagents to α,β-Unsaturated Esters. Tetrahedron: Asymmetry 1999, 10, 4047–4056. 10.1016/S0957-4166(99)00417-6. - DOI
- Sakuma S.; Sakai M.; Itooka R.; Miyaura N. Asymmetric Conjugate 1,4-Addition of Arylboronic Acids to α,β-Unsaturated Esters Catalyzed by Rhodium(I)/(S)-Binap. J. Org. Chem. 2000, 65, 5951–5955. 10.1021/jo0002184. - DOI - PubMed
LinkOut - more resources
Full Text Sources

