Duration of medication treatment for opioid-use disorder and risk of overdose among Medicaid enrollees in 11 states: a retrospective cohort study
- PMID: 35652681
- PMCID: PMC10683938
- DOI: 10.1111/add.15959
Duration of medication treatment for opioid-use disorder and risk of overdose among Medicaid enrollees in 11 states: a retrospective cohort study
Abstract
Background and aims: Medication for opioid use disorder (MOUD) reduces harms associated with opioid use disorder (OUD), including risk of overdose. Understanding how variation in MOUD duration influences overdose risk is important as health-care payers increasingly remove barriers to treatment continuation (e.g. prior authorization). This study measured the association between MOUD continuation, relative to discontinuation, and opioid-related overdose among Medicaid beneficiaries.
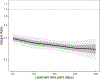
Design: Retrospective cohort study using landmark survival analysis. We estimated the association between treatment continuation and overdose risk at 5 points after the index, or first, MOUD claim. Censoring events included death and disenrollment.
Setting and participants: Medicaid programs in 11 US states: Delaware, Kentucky, Maryland, Maine, Michigan, North Carolina, Ohio, Pennsylvania, Virginia, West Virginia and Wisconsin. A total of 293 180 Medicaid beneficiaries aged 18-64 years with a diagnosis of OUD and had a first MOUD claim between 2016 and 2017.
Measurements: MOUD formulations included methadone, buprenorphine and naltrexone. We measured medically treated opioid-related overdose within claims within 12 months of the index MOUD claim.
Findings: Results were consistent across states. In pooled results, 5.1% of beneficiaries had an overdose, and 67% discontinued MOUD before an overdose or censoring event within 12 months. Beneficiaries who continued MOUD beyond 60 days had a lower relative overdose hazard ratio (HR) compared with those who discontinued by day 60 [HR = 0.39; 95% confidence interval (CI) = 0.36-0.42; P < 0.0001]. MOUD continuation was associated with lower overdose risk at 120 days (HR = 0.34; 95% CI = 0.31-0.37; P < 0.0001), 180 days (HR = 0.31; 95% CI = 0.29-0.34; P < 0.0001), 240 days (HR = 0.29; 95% CI = 0.26-0.31; P < 0.0001) and 300 days (HR = 0.28; 95% CI = 0.24-0.32; P < 0.0001). The hazard of overdose was 10% lower with each additional 60 days of MOUD (95% CI = 0.88-0.92; P < 0.0001).
Conclusions: Continuation of medication for opioid use disorder (MOUD) in US Medicaid beneficiaries was associated with a substantial reduction in overdose risk up to 12 months after the first claim for MOUD.
Keywords: Distributed research network; Medicaid; landmark survival analysis; medication; opioid use disorder; opioid-related overdose.
© 2022 Society for the Study of Addiction.
Conflict of interest statement
Conflict of interest declaration: None
Figures
Comment in
-
Commentary on Burns et al: MOUD saves lives, especially after 60 days, and the longer the better.Addiction. 2022 Dec;117(12):3089-3090. doi: 10.1111/add.16043. Epub 2022 Sep 13. Addiction. 2022. PMID: 36100579 Free PMC article.
References
-
- Ahmad FB Rossen LM, Sutton P. Provisional drug overdose death counts. Hyattsville, MD: National Center for Health Statistics; 2021
-
- Johnson RE, Jaffe JH, Fudala PJ. A controlled trial of buprenorphine treatment for opioid dependence. JAMA. 1992;267(20):2750–2755. - PubMed
-
- Ling W, Wesson DR, Charuvastra C, Klett CJ. A controlled trial comparing buprenorphine and methadone maintenance in opioid dependence. Arch Gen Psychiatry. 1996;53(5):401–407. - PubMed
-
- Strain EC, Bigelow GE, Liebson IA, Stitzer ML. Moderate- vs high-dose methadone in the treatment of opioid dependence - A randomized trial. JAMA. 1999;281(11):1000–1005. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

