Intravitreal aflibercept for diabetic macular edema in real-world clinical practice in Japan: 24-month outcomes
- PMID: 35652946
- PMCID: PMC9581854
- DOI: 10.1007/s00417-022-05703-9
Intravitreal aflibercept for diabetic macular edema in real-world clinical practice in Japan: 24-month outcomes
Erratum in
-
Correction to: Intravitreal aflibercept for diabetic macular edema in real‑world clinical practice in Japan: 24‑month outcomes.Graefes Arch Clin Exp Ophthalmol. 2023 Jan;261(1):283-287. doi: 10.1007/s00417-022-05917-x. Graefes Arch Clin Exp Ophthalmol. 2023. PMID: 36472690 Free PMC article. No abstract available.
Abstract
Purpose: To report the safety and effectiveness of intravitreal aflibercept (IVT-AFL) for diabetic macular edema (DME) in the real-world clinical practice setting in Japan.
Methods: In this prospective, multicenter, observational, post-marketing surveillance, patients with DME newly receiving IVT-AFL were enrolled. During a 24-month follow-up, the primary outcome was the occurrence of safety events. Other pre-specified endpoints were effectiveness indicators, such as best-corrected visual acuity (BCVA), central retinal thickness, and injection frequency.
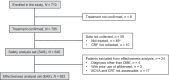
Results: In total, 646 patients administered at least one IVT-AFL injection were included in the safety analysis. During the follow-up period, adverse events occurred in 42 patients (6.50%), whereas adverse drug reactions occurred in 12 (1.86%). In the 12 patients who had adverse drug reactions, seven events occurred in seven patients within the first month of the most recent injection. In addition, 622 patients were included in the effectiveness analysis set. The number of injections over 24 months was 3.6 ± 3.0 (mean ± standard deviation [SD]). BCVA (logarithm of the minimum angle of resolution) was 0.437 ± 0.362 (mean ± SD) (n = 622) at baseline and 0.321 ± 0.348 (n = 177) after 24 months of treatment with IVT-AFL. Central retinal thickness was 440.8 ± 134.2 μm (mean ± SD) (n = 444) at baseline and 355.5 ± 126.4 μm (n = 140) at 24 months.
Conclusion: Routine administration of IVT-AFL for DME was not associated with new safety concerns, and BCVA outcomes were maintained over 24 months in the real-world setting. Nonetheless, patients in this real-world setting received fewer injections than those in clinical trials, suggesting that a margin for improvement exists in clinical practice.
Trial registration: ClinicalTrials.gov: NCT02425501.
Keywords: Anti-vascular endothelial growth factor treatment; Diabetic macular edema; Intravitreal aflibercept; Real-world clinical practice.
© 2022. The Author(s).
Conflict of interest statement
MS received research funding form Novartis, Chugai, Alcon, and Bayer; personal fees or support from Novartis, Alcon, Santen, Kowa, Senju, Bayer, and Wakamoto outside the submitted work. MK received research funding from Novartis, Alcon, Santen, Otsuka, Senju, HOYA, Pfizer, AMO, and Polish; consulting fees from Senju and Ono; personal fees or support from Novartis, Alcon, Santen, Sanofi, Kowa, Otsuka, Senju, Nidek, Bayer, Ono, Sanwa Kagaku, and Chuo Sangyo Boeki outside the submitted work. Although MK is an editorial board member of the journal, there was no involvement with the peer review process for this article. CH, KH, and TS are employees of Bayer Yakuhin, Ltd.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

