Synthesis of Pleuromutilin
- PMID: 35653288
- PMCID: PMC10122272
- DOI: 10.1021/jacs.2c04708
Synthesis of Pleuromutilin
Abstract
Synthesis of a potent inhibitor of bacterial protein synthesis, pleuromutilin, is described. Assembly of the critical cyclooctane fragment relies on an oxidative ring-expansion, and complete stereochemical relay in the synthetic sequence is enabled by the judicious choice of tactics. The requisite connectivity pattern of the perhydroindanone motif is rapidly established in a sequence of cycloaddition and radical cyclization events. Application of this strategy allows for preparation of the target natural product in 16 steps from commercially available material.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
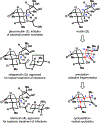

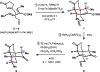

References
-
- Poulsen SM; Karlsson M; Johansson LB; Vester B The pleuromutilin drugs tiamulin and valnemulin bind to the RNA at the peptidyl transferase centre on the ribosome. Mol. Microbiol 2001, 41, 1091. - PubMed
-
- (a)Anchel M Chemical studies with pleuromutilin. J. Biol. Chem 1952, 199, 133. - PubMed
- (b) Arigoni D Structure of a new type of terpene. Gazz. Chim. Ital 1962, 92, 884.
- (c) Birch AJ; Holzapfel CW; Rickards RW The structure and some aspects of the biosynthesis of pleuromoutilin. Tetrahedron 1966, 22, 359.
- For X-ray crystallographic studies see: (d) Dobler M; Duerr BG Mono-O-bromoacetylpleuromutilin. Cryst. Struct. Comm 1975, 4, 259.

