PHACTR1 modulates vascular compliance but not endothelial function: a translational study
- PMID: 35653516
- PMCID: PMC10064844
- DOI: 10.1093/cvr/cvac092
PHACTR1 modulates vascular compliance but not endothelial function: a translational study
Abstract
Aims: The non-coding locus at 6p24 located in Intron 3 of PHACTR1 has consistently been implicated as a risk allele in myocardial infarction and multiple other vascular diseases. Recent murine studies have identified a role for Phactr1 in the development of atherosclerosis. However, the role of PHACTR1 in vascular tone and in vivo vascular remodelling has yet to be established. The aim of this study was to investigate the role of PHACTR1 in vascular function.
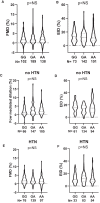
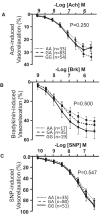
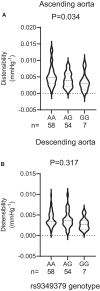
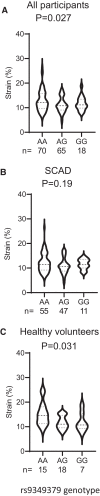
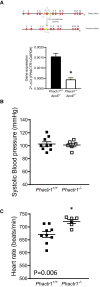
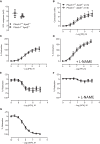
Methods and results: Prospectively recruited coronary artery disease (CAD) patients undergoing bypass surgery and retrospectively recruited spontaneous coronary artery dissection (SCAD) patients and matched healthy volunteers were genotyped at the PHACTR1 rs9349379 locus. We observed a significant association between the PHACTR1 loci and changes in distensibility in both the ascending aorta (AA = 0.0053 ± 0.0004, AG = 0.0041 ± 0.003, GG = 0.0034 ± 0.0009, P < 0.05, n = 58, 54, and 7, respectively) and carotid artery (AA = 12.83 ± 0.51, AG = 11.14 ± 0.38, GG = 11.69 ± 0.66, P < 0.05, n = 70, 65, and 18, respectively). This association was not observed in the descending aorta or in SCAD patients. In contrast, the PHACTR1 locus was not associated with changes in endothelial cell function with no association between the rs9349379 locus and in vivo or ex vivo vascular function observed in CAD patients. This finding was confirmed in our murine model where the loss of Phactr1 on the pro-atherosclerosis ApoE-/- background did not alter ex vivo vascular function.
Conclusion: In conclusion, we have shown a role for PHACTR1 in arterial compliance across multiple vascular beds. Our study suggests that PHACTR1 has a key structural role within the vasculature.
Keywords: PHACTR1; Arteries; Compliance; Endothelial cells.
© The Author(s) 2022. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: D.A. reports in kind support from Astra Zeneca Inc. for SCAD genetics research and research funding from Astra Zeneca for unrelated research. He has received educational funding from Abbott Vascular Inc. to support a clinical research fellow. He has conducted consultancy for General Electric Inc. to support general research funds. No other authors have any relationships to declare.
Figures
Similar articles
-
Myocardial Infarction-Associated SNP at 6p24 Interferes With MEF2 Binding and Associates With PHACTR1 Expression Levels in Human Coronary Arteries.Arterioscler Thromb Vasc Biol. 2015 Jun;35(6):1472-1479. doi: 10.1161/ATVBAHA.115.305534. Epub 2015 Apr 2. Arterioscler Thromb Vasc Biol. 2015. PMID: 25838425 Free PMC article.
-
PHACTR1 splicing isoforms and eQTLs in atherosclerosis-relevant human cells.BMC Med Genet. 2018 Jun 8;19(1):97. doi: 10.1186/s12881-018-0616-7. BMC Med Genet. 2018. PMID: 29884117 Free PMC article.
-
Genetic and environmental risk factors for atherosclerosis regulate transcription of phosphatase and actin regulating gene PHACTR1.Atherosclerosis. 2016 Jul;250:95-105. doi: 10.1016/j.atherosclerosis.2016.04.025. Epub 2016 May 2. Atherosclerosis. 2016. PMID: 27187934 Free PMC article.
-
The association of polymorphism in PHACTR1 rs9349379 and rs12526453 with coronary artery atherosclerosis or coronary artery calcification. A systematic review.Coron Artery Dis. 2021 Aug 1;32(5):448-458. doi: 10.1097/MCA.0000000000000942. Coron Artery Dis. 2021. PMID: 33660664
-
From Atherosclerosis to Spontaneous Coronary Artery Dissection: Defining a Clinical and Genetic Risk Spectrum for Myocardial Infarction.Curr Atheroscler Rep. 2024 Jul;26(7):331-340. doi: 10.1007/s11883-024-01208-4. Epub 2024 May 18. Curr Atheroscler Rep. 2024. PMID: 38761354 Review.
Cited by
-
Intra-operative and post-operative management of conduits for coronary artery bypass grafting: a clinical consensus statement of the European Society of Cardiology Working Group on Cardiovascular Surgery and the European Association for Cardio-Thoracic Surgery Coronary Task Force.Eur Heart J. 2025 Jan 3;46(1):19-34. doi: 10.1093/eurheartj/ehae654. Eur Heart J. 2025. PMID: 39412205 Free PMC article. Review.
-
Intra-operative and post-operative management of conduits for coronary artery bypass grafting: a clinical consensus statement of the European Society of Cardiology Working Group on Cardiovascular Surgery and the European Association for Cardio-Thoracic Surgery Coronary Task Force.Eur J Cardiothorac Surg. 2024 Nov 28;66(6):ezae400. doi: 10.1093/ejcts/ezae400. Eur J Cardiothorac Surg. 2024. PMID: 39656609 Free PMC article. Review.
-
Endothelial PHACTR1 Promotes Endothelial Activation and Atherosclerosis by Repressing PPARγ Activity Under Disturbed Flow in Mice.Arterioscler Thromb Vasc Biol. 2023 Aug;43(8):e303-e322. doi: 10.1161/ATVBAHA.122.318173. Epub 2023 May 18. Arterioscler Thromb Vasc Biol. 2023. PMID: 37199156 Free PMC article.
-
PHACTR1, a coronary artery disease risk gene, mediates endothelial dysfunction.Front Immunol. 2022 Aug 25;13:958677. doi: 10.3389/fimmu.2022.958677. eCollection 2022. Front Immunol. 2022. PMID: 36091033 Free PMC article.
-
PHACTR1 and Atherosclerosis: It's Complicated.Arterioscler Thromb Vasc Biol. 2023 Aug;43(8):1409-1411. doi: 10.1161/ATVBAHA.123.319545. Epub 2023 Jun 15. Arterioscler Thromb Vasc Biol. 2023. PMID: 37317846 Free PMC article. No abstract available.
References
-
- Nikpay M, Goel A, Won HH, Hall LM, Willenborg C, Kanoni S, Saleheen D, Kyriakou T, Nelson CP, Hopewell JC, Webb TR, Zeng L, Dehghan A, Alver M, Armasu SM, Auro K, Bjonnes A, Chasman DI, Chen S, Ford I, Franceschini N, Gieger C, Grace C, Gustafsson S, Huang J, Hwang SJ, Kim YK, Kleber ME, Lau KW, Lu X, Lu Y, Lyytikainen LP, Mihailov E, Morrison AC, Pervjakova N, Qu L, Rose LM, Salfati E, Saxena R, Scholz M, Smith AV, Tikkanen E, Uitterlinden A, Yang X, Zhang W, Zhao W, de Andrade M, de Vries PS, van Zuydam NR, Anand SS, Bertram L, Beutner F, Dedoussis G, Frossard P, Gauguier D, Goodall AH, Gottesman O, Haber M, Han BG, Huang J, Jalilzadeh S, Kessler T, Konig IR, Lannfelt L, Lieb W, Lind L, Lindgren CM, Lokki ML, Magnusson PK, Mallick NH, Mehra N, Meitinger T, Memon FU, Morris AP, Nieminen MS, Pedersen NL, Peters A, Rallidis LS, Rasheed A, Samuel M, Shah SH, Sinisalo J, Stirrups KE, Trompet S, Wang L, Zaman KS, Ardissino D, Boerwinkle E, Borecki IB, Bottinger EP, Buring JE, Chambers JC, Collins R, Cupples LA, Danesh J, Demuth I, Elosua R, Epstein SE, Esko T, Feitosa MF, Franco OH, Franzosi MG, Granger CB, Gu D, Gudnason V, Hall AS, Hamsten A, Harris TB, Hazen SL, Hengstenberg C, Hofman A, Ingelsson E, Iribarren C, Jukema JW, Karhunen PJ, Kim BJ, Kooner JS, Kullo IJ, Lehtimaki T, Loos RJ, Melander O, Metspalu A, Marz W, Palmer CN, Perola M, Quertermous T, Rader DJ, Ridker PM, Ripatti S, Roberts R, Salomaa V, Sanghera DK, Schwartz SM, Seedorf U, Stewart AF, Stott DJ, Thiery J, Zalloua PA, O’Donnell CJ, Reilly MP, Assimes TL, Thompson JR, Erdmann J, Clarke R, Watkins H, Kathiresan S, McPherson R, Deloukas P, Schunkert H, Samani NJ, Farrall M, Consortium CAD. A comprehensive 1,000 genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet 2015;47:1121–1130. - PMC - PubMed
-
- Howson JMM, Zhao W, Barnes DR, Ho WK, Young R, Paul DS, Waite LL, Freitag DF, Fauman EB, Salfati EL, Sun BB, Eicher JD, Johnson AD, Sheu WHH, Nielsen SF, Lin WY, Surendran P, Malarstig A, Wilk JB, Tybjærg-Hansen A, Rasmussen KL, Kamstrup PR, Deloukas P, Erdmann J, Kathiresan S, Samani NJ, Schunkert H, Watkins H, Do R, Rader DJ, Johnson JA, Hazen SL, Quyyumi AA, Spertus JA, Pepine CJ, Franceschini N, Justice A, Reiner AP, Buyske S, Hindorff LA, Carty CL, North KE, Kooperberg C, Boerwinkle E, Young K, Graff M, Peters U, Absher D, Hsiung CA, Lee WJ, Taylor KD, Chen YH, Lee IT, Guo X, Chung RH, Hung YJ, Rotter JI, Juang JJ, Quertermous T, Wang TD, Rasheed A, Frossard P, Alam DS, Majumder AAS, Di Angelantonio E, Chowdhury R, Chen YI, Nordestgaard BG, Assimes TL, Danesh J, Butterworth AS, Saleheen D. Fifteen new risk loci for coronary artery disease highlight arterial-wall-specific mechanisms. Nat Genet 2017;49:1113–1119. - PMC - PubMed
-
- Deloukas P, Kanoni S, Willenborg C, Farrall M, Assimes TL, Thompson JR, Ingelsson E, Saleheen D, Erdmann J, Goldstein BA, Stirrups K, König IR, Cazier JB, Johansson A, Hall AS, Lee JY, Willer CJ, Chambers JC, Esko T, Folkersen L, Goel A, Grundberg E, Havulinna AS, Ho WK, Hopewell JC, Eriksson N, Kleber ME, Kristiansson K, Lundmark P, Lyytikäinen LP, Rafelt S, Shungin D, Strawbridge RJ, Thorleifsson G, Tikkanen E, Van Zuydam N, Voight BF, Waite LL, Zhang W, Ziegler A, Absher D, Altshuler D, Balmforth AJ, Barroso I, Braund PS, Burgdorf C, Claudi-Boehm S, Cox D, Dimitriou M, Do R, Doney AS, El Mokhtari N, Eriksson P, Fischer K, Fontanillas P, Franco-Cereceda A, Gigante B, Groop L, Gustafsson S, Hager J, Hallmans G, Han BG, Hunt SE, Kang HM, Illig T, Kessler T, Knowles JW, Kolovou G, Kuusisto J, Langenberg C, Langford C, Leander K, Lokki ML, Lundmark A, McCarthy MI, Meisinger C, Melander O, Mihailov E, Maouche S, Morris AD, Müller-Nurasyid M, Nikus K, Peden JF, Rayner NW, Rasheed A, Rosinger S, Rubin D, Rumpf MP, Schäfer A, Sivananthan M, Song C, Stewart AF, Tan ST, Thorgeirsson G, van der Schoot CE, Wagner PJ, Wells GA, Wild PS, Yang TP, Amouyel P, Arveiler D, Basart H, Boehnke M, Boerwinkle E, Brambilla P, Cambien F, Cupples AL, de Faire U, Dehghan A, Diemert P, Epstein SE, Evans A, Ferrario MM, Ferrières J, Gauguier D, Go AS, Goodall AH, Gudnason V, Hazen SL, Holm H, Iribarren C, Jang Y, Kähönen M, Kee F, Kim HS, Klopp N, Koenig W, Kratzer W, Kuulasmaa K, Laakso M, Laaksonen R, Lee JY, Lind L, Ouwehand WH, Parish S, Park JE, Pedersen NL, Peters A, Quertermous T, Rader DJ, Salomaa V, Schadt E, Shah SH, Sinisalo J, Stark K, Stefansson K, Trégouët DA, Virtamo J, Wallentin L, Wareham N, Zimmermann ME, Nieminen MS, Hengstenberg C, Sandhu MS, Pastinen T, Syvänen AC, Hovingh GK, Dedoussis G, Franks PW, Lehtimäki T, Metspalu A, Zalloua PA, Siegbahn A, Schreiber S, Ripatti S, Blankenberg SS, Perola M, Clarke R, Boehm BO, O’Donnell C, Reilly MP, März W, Collins R, Kathiresan S, Hamsten A, Kooner JS, Thorsteinsdottir U, Danesh J, Palmer CN, Roberts R, Watkins H, Schunkert H, Samani NJ. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet 2013;45:25–33. - PMC - PubMed
-
- Beaudoin M, Gupta RM, Won HH, Lo KS, Do R, Henderson CA, Lavoie-St-Amour C, Langlois S, Rivas D, Lehoux S, Kathiresan S, Tardif JC, Musunuru K, Lettre G. Myocardial infarction-associated SNP at 6p24 interferes with MEF2 binding and associates with PHACTR1 expression levels in human coronary arteries. Arterioscler Thromb Vasc Biol 2015;35:1472–1479. - PMC - PubMed
-
- Ford TJ, Corcoran D, Padmanabhan S, Aman A, Rocchiccioli P, Good R, McEntegart M, Maguire JJ, Watkins S, Eteiba H, Shaukat A, Lindsay M, Robertson K, Hood S, McGeoch R, McDade R, Yii E, Sattar N, Hsu LY, Arai AE, Oldroyd KG, Touyz RM, Davenport AP, Berry C. Genetic dysregulation of endothelin-1 is implicated in coronary microvascular dysfunction. Eur Heart J 2020;41:3239–3252. - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- RG/12/5/29576/BHF_/British Heart Foundation/United Kingdom
- RG/F/22/110085/BHF_/British Heart Foundation/United Kingdom
- CH/F/21/90009/BHF_/British Heart Foundation/United Kingdom
- RG/F/21/110040/BHF_/British Heart Foundation/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- RG/17/10/32859/BHF_/British Heart Foundation/United Kingdom
- SP/19/2/34462/BHF_/British Heart Foundation/United Kingdom
- PG/13/96/30608/BHF_/British Heart Foundation/United Kingdom
- CH/16/1/32013/BHF_/British Heart Foundation/United Kingdom
- PG/20/10066/BHF_/British Heart Foundation/United Kingdom
- PG/15/35/31403/BHF_/British Heart Foundation/United Kingdom
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

