Degradable Polyphosphoramidate via Ring-Opening Metathesis Polymerization
- PMID: 35653670
- PMCID: PMC11042488
- DOI: 10.1021/acsmacrolett.0c00401
Degradable Polyphosphoramidate via Ring-Opening Metathesis Polymerization
Abstract
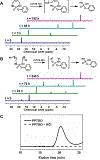
We report the synthesis of a degradable polyphosphoramidate via ring-opening metathesis polymerization (ROMP) with the Grubbs initiator (IMesH2)(C5H5N)2(Cl)2Ru═CHPh. Controlled ROMP of a low ring strain diazaphosphepine-based cyclic olefin was achieved at low temperatures to afford well-defined polymers that readily undergo degradation in acidic conditions via the cleavage of the acid-labile phosphoramidate linkages. The diazaphosphepine monomer was compatible in random and block copolymerizations with phenyl and oligo(ethylene glycol) bearing norbornenes. This approach introduced partial or complete degradability into the polymer backbones. With this chemistry, we accessed amphiphilic poly(diazaphosphepine-norbornene) copolymers that could be used to prepare micellar nanoparticles.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
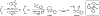
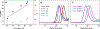

References
-
- Delplace V; Nicolas J Degradable vinyl polymers for biomedical applications. Nat. Chem. 2015, 7, 771–784. - PubMed
-
- Hillmyer MA; Tolman WB Aliphatic Polyester Block Polymers: Renewable, Degradable, and Sustainable. Acc. Chem. Res. 2014, 47, 2390–2396. - PubMed
-
- Semsarilar M; Perrier S ‘Green’ reversible addition-fragmentation chain-transfer (RAFT) polymerization. Nat. Chem. 2010, 2, 811–820. - PubMed
-
- Farah S; Anderson DG; Langer R Physical and mechanical properties of PLA, and their functions in widespread applications - A comprehensive review. Adv. Drug Delivery Rev. 2016, 107, 367–392. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

