A journey from phosphotyrosine to phosphohistidine and beyond
- PMID: 35654043
- PMCID: PMC9219344
- DOI: 10.1016/j.molcel.2022.05.007
A journey from phosphotyrosine to phosphohistidine and beyond
Abstract
Protein phosphorylation is a reversible post-translational modification. Nine of the 20 natural amino acids in proteins can be phosphorylated, but most of what we know about the roles of protein phosphorylation has come from studies of serine, threonine, and tyrosine phosphorylation. Much less is understood about the phosphorylation of histidine, lysine, arginine, cysteine, aspartate, and glutamate, so-called non-canonical phosphorylations. Phosphohistidine (pHis) was discovered 60 years ago as a mitochondrial enzyme intermediate; since then, evidence for the existence of histidine kinases and phosphohistidine phosphatases has emerged, together with examples where protein function is regulated by reversible histidine phosphorylation. pHis is chemically unstable and has thus been challenging to study. However, the recent development of tools for studying pHis has accelerated our understanding of the multifaceted functions of histidine phosphorylation, revealing a large number of proteins that are phosphorylated on histidine and implicating pHis in a wide range of cellular processes.
Keywords: histidine kinase; phosphohistidine; phosphohistidine phosphatase.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The author holds a patent describing the generation and use of phosphohistidine-specific monoclonal antibodies. The author is a member of the Molecular Cell advisory board.
Figures

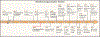
References
-
- Adam K, Fuhs S, Meisenhelder J, Aslanian A, Diedrich J, Moresco J, La Clair J, Yates JR III, and Hunter T (2019). A non-acidic method using hydroxyapatite and phosphohistidine monoclonal antibodies allows enrichment of phosphopeptides containing non-conventional phosphorylations for mass spectrometry analysis. bioRxiv, doi: 10.1101/691352. - DOI
-
- Alonso A, Sasin J, Bottini N, Friedberg I, Friedberg I, Osterman A, Godzik A, Hunter T, Dixon J, and Mustelin T (2004). Protein tyrosine phosphatases in the human genome. Cell 117, :699–711. - PubMed
-
- Anderson JW, Waygood EB, Saier JMH, and Reizer J (1992). Properties of phosphorylated protein intermediates of the bacterial phosphotransferase system. Biochem. Cell Biol 70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

