Developmental organization of neural dynamics supporting auditory perception
- PMID: 35654375
- PMCID: PMC9354710
- DOI: 10.1016/j.neuroimage.2022.119342
Developmental organization of neural dynamics supporting auditory perception
Abstract
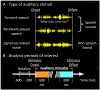
Purpose: A prominent view of language acquisition involves learning to ignore irrelevant auditory signals through functional reorganization, enabling more efficient processing of relevant information. Yet, few studies have characterized the neural spatiotemporal dynamics supporting rapid detection and subsequent disregard of irrelevant auditory information, in the developing brain. To address this unknown, the present study modeled the developmental acquisition of cost-efficient neural dynamics for auditory processing, using intracranial electrocorticographic responses measured in individuals receiving standard-of-care treatment for drug-resistant, focal epilepsy. We also provided evidence demonstrating the maturation of an anterior-to-posterior functional division within the superior-temporal gyrus (STG), which is known to exist in the adult STG.
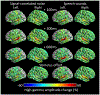
Methods: We studied 32 patients undergoing extraoperative electrocorticography (age range: eight months to 28 years) and analyzed 2,039 intracranial electrode sites outside the seizure onset zone, interictal spike-generating areas, and MRI lesions. Patients were given forward (normal) speech sounds, backward-played speech sounds, and signal-correlated noises during a task-free condition. We then quantified sound processing-related neural costs at given time windows using high-gamma amplitude at 70-110 Hz and animated the group-level high-gamma dynamics on a spatially normalized three-dimensional brain surface. Finally, we determined if age independently contributed to high-gamma dynamics across brain regions and time windows.
Results: Group-level analysis of noise-related neural costs in the STG revealed developmental enhancement of early high-gamma augmentation and diminution of delayed augmentation. Analysis of speech-related high-gamma activity demonstrated an anterior-to-posterior functional parcellation in the STG. The left anterior STG showed sustained augmentation throughout stimulus presentation, whereas the left posterior STG showed transient augmentation after stimulus onset. We found a double dissociation between the locations and developmental changes in speech sound-related high-gamma dynamics. Early left anterior STG high-gamma augmentation (i.e., within 200 ms post-stimulus onset) showed developmental enhancement, whereas delayed left posterior STG high-gamma augmentation declined with development.
Conclusions: Our observations support the model that, with age, the human STG refines neural dynamics to rapidly detect and subsequently disregard uninformative acoustic noises. Our study also supports the notion that the anterior-to-posterior functional division within the left STG is gradually strengthened for efficient speech-sound perception after birth.
Keywords: Electrocorticography (ECoG); Event-related high-gamma synchronization; Intracranial electroencephalography (EEG) recording; Language acquisition; Neural pruning; Neurolinguistics; Ontogeny; Pediatric epilepsy surgery; Physiological high-frequency oscillations (HFOs); Subdural grid electrodes.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors have no conflicts of interest to report. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Figures






References
-
- Bilecen D, Seifritz E, Scheffler K, Henning J, Schulte AC, 2002. Amplitopicity of the human auditory cortex: an fMRI study. Neuroimage 17, 710–718 . - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

