Self-Reported and Physiologic Reactions to Third BNT162b2 mRNA COVID-19 (Booster) Vaccine Dose
- PMID: 35654410
- PMCID: PMC9239876
- DOI: 10.3201/eid2807.212330
Self-Reported and Physiologic Reactions to Third BNT162b2 mRNA COVID-19 (Booster) Vaccine Dose
Abstract
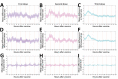
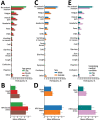
Despite extensive technological advances in recent years, objective and continuous assessment of physiologic measures after vaccination is rarely performed. We conducted a prospective observational study to evaluate short-term self-reported and physiologic reactions to the booster BNT162b2 mRNA (Pfizer-BioNTech, https://www.pfizer.com) vaccine dose. A total of 1,609 participants were equipped with smartwatches and completed daily questionnaires through a dedicated mobile application. The extent of systemic reactions reported after the booster dose was similar to that of the second dose and considerably greater than that of the first dose. Analyses of objective heart rate and heart rate variability measures recorded by smartwatches further supported this finding. Subjective and objective reactions after the booster dose were more apparent in younger participants and in participants who did not have underlying medical conditions. Our findings further support the safety of the booster dose from subjective and objective perspectives and underscore the need for integrating wearables in clinical trials.
Keywords: BNT162b2; COVID-19; SARS-CoV-2; adverse effects; booster vaccine; coronavirus disease; coronaviruses; heart rate variability; mRNA; physiologic reactions; respiratory infections; self-reported reactions; severe acute respiratory syndrome coronavirus 2; smartwatch; third COVID-19 dose; vaccine; viruses; wearables; zoonoses.
Figures


Similar articles
-
Safety of the fourth COVID-19 BNT162b2 mRNA (second booster) vaccine: a prospective and retrospective cohort study.Lancet Respir Med. 2023 Feb;11(2):139-150. doi: 10.1016/S2213-2600(22)00407-6. Epub 2022 Nov 18. Lancet Respir Med. 2023. PMID: 36410364 Free PMC article.
-
Association of a Third Dose of BNT162b2 Vaccine With Incidence of SARS-CoV-2 Infection Among Health Care Workers in Israel.JAMA. 2022 Jan 25;327(4):341-349. doi: 10.1001/jama.2021.23641. JAMA. 2022. PMID: 35006256 Free PMC article.
-
COVID-19 vaccine waning and effectiveness and side-effects of boosters: a prospective community study from the ZOE COVID Study.Lancet Infect Dis. 2022 Jul;22(7):1002-1010. doi: 10.1016/S1473-3099(22)00146-3. Epub 2022 Apr 8. Lancet Infect Dis. 2022. PMID: 35405090 Free PMC article.
-
Specific Anti-SARS-CoV-2 Humoral and Cellular Immune Responses After Booster Dose of BNT162b2 Pfizer-BioNTech mRNA-Based Vaccine: Integrated Study of Adaptive Immune System Components.Front Immunol. 2022 Mar 24;13:856657. doi: 10.3389/fimmu.2022.856657. eCollection 2022. Front Immunol. 2022. PMID: 35401503 Free PMC article.
-
Safety Monitoring of COVID-19 Vaccine Booster Doses Among Persons Aged 12-17 Years - United States, December 9, 2021-February 20, 2022.MMWR Morb Mortal Wkly Rep. 2022 Mar 4;71(9):347-351. doi: 10.15585/mmwr.mm7109e2. MMWR Morb Mortal Wkly Rep. 2022. PMID: 35239637 Free PMC article.
Cited by
-
Preclinical evaluation of AGT mRNA replacement therapy for primary hyperoxaluria type I disease.Sci Adv. 2025 Apr 11;11(15):eadt9694. doi: 10.1126/sciadv.adt9694. Epub 2025 Apr 9. Sci Adv. 2025. PMID: 40203111 Free PMC article.
-
A comprehensive review of SARS-CoV-2 vaccines: Pfizer, Moderna & Johnson & Johnson.Hum Vaccin Immunother. 2022 Dec 31;18(1):2002083. doi: 10.1080/21645515.2021.2002083. Epub 2022 Feb 7. Hum Vaccin Immunother. 2022. PMID: 35130825 Free PMC article. Review.
-
Wearable Devices and Explainable Unsupervised Learning for COVID-19 Detection and Monitoring.Diagnostics (Basel). 2023 Sep 28;13(19):3071. doi: 10.3390/diagnostics13193071. Diagnostics (Basel). 2023. PMID: 37835814 Free PMC article.
-
Higher sensitivity monitoring of reactions to COVID-19 vaccination using smartwatches.NPJ Digit Med. 2022 Sep 9;5(1):140. doi: 10.1038/s41746-022-00683-w. NPJ Digit Med. 2022. PMID: 36085312 Free PMC article.
-
Strong Humoral but Not Cellular Immune Responses against SARS-CoV-2 in Individuals with Oncohematological Disease Who Were Treated with Rituximab before Receiving a Vaccine Booster.Cancers (Basel). 2022 Nov 10;14(22):5537. doi: 10.3390/cancers14225537. Cancers (Basel). 2022. PMID: 36428631 Free PMC article.
References
-
- Kupferschmidt K, Wadman M. Delta variant triggers new phase in the pandemic. Science. 2021;372:1375–6. 10.1126/science.372.6549.1375 - DOI
-
- World Health Organization. Tracking SARS-CoV-2 variants [cited 2022 Apr 13]. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants - PubMed
-
- Mlcochova P, Kemp SA, Dhar MS, Papa G, Meng B, Ferreira IATM, et al.; Indian SARS-CoV-2 Genomics Consortium (INSACOG); Genotype to Phenotype Japan (G2P-Japan) Consortium; CITIID-NIHR BioResource COVID-19 Collaboration. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature. 2021;599:114–9. 10.1038/s41586-021-03944-y - DOI - PMC - PubMed
-
- Centers for Disease Control and Prevention. Delta variant: what we know about the science [cited 2022 Apr 13]. https://stacks.cdc.gov/view/cdc/108671
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

