Effect of tofacitinib on pain, fatigue, health-related quality of life and work productivity in patients with active ankylosing spondylitis: results from a phase III, randomised, double-blind, placebo-controlled trial
- PMID: 35654457
- PMCID: PMC9163535
- DOI: 10.1136/rmdopen-2022-002253
Effect of tofacitinib on pain, fatigue, health-related quality of life and work productivity in patients with active ankylosing spondylitis: results from a phase III, randomised, double-blind, placebo-controlled trial
Abstract
Background: Ankylosing spondylitis (AS) impacts quality of life. We assessed patient-reported outcomes (PROs), pain, fatigue, health-related quality of life (HRQoL) and work productivity in a phase III trial of tofacitinib.
Methods: Adults with AS and with inadequate response/intolerance to ≥2 non-steroidal anti-inflammatory drugs received tofacitinib 5 mg twice daily or placebo for 16 weeks. Afterwards, all received open-label tofacitinib until week 48. Change from baseline to week 48 was determined for PROs: total back pain; nocturnal spinal pain; Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) overall spinal pain (Q2); Functional Assessment of Chronic Illness Therapy-Fatigue; BASDAI fatigue (Q1); AS Quality of Life (ASQoL); Short Form-36 Health Survey Version 2 (SF-36v2); EuroQoL-Five Dimension-Three Level health profile and Visual Analogue Scale; and the Work Productivity and Activity Impairment (WPAI) questionnaire. Improvements from baseline ≥minimum clinically important difference, and scores ≥normative values at week 16 were evaluated.
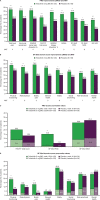
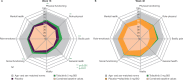
Results: In 269 randomised and treated patients, at week 16, there were greater least squares mean improvements from baseline with tofacitinib 5 mg twice daily versus placebo in BASDAI overall spinal pain (-2.85 vs -1.34), BASDAI fatigue (-2.36 vs -1.08), ASQoL (-4.03 vs -2.01) and WPAI overall work impairment (-21.49 vs -7.64) (all p<0.001); improvements continued/increased to week 48. Improved spinal pain with tofacitinib was seen by week 2. Patients receiving tofacitinib reported clinically meaningful PRO improvements at week 16. Percentages with PRO scores ≥normative values at week 16 were greater with tofacitinib in SF-36v2 Physical Component Summary, physical functioning and bodily pain domains (p≤0.05).
Conclusions: In patients with AS, treatment with tofacitinib 5 mg twice daily resulted in clinically meaningful improvements in pain, fatigue, HRQoL and work productivity versus placebo to week 16, which were sustained to week 48.
Trial registration number: NCT03502616.
Keywords: Antirheumatic Agents; Inflammation; Patient Reported Outcome Measures.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: VN-C has received research/grant support from AbbVie and Novartis, and is a member of the speakers’ bureaus for AbbVie, Eli Lilly, Janssen, Moonlake, MSD, Novartis, Pfizer Inc, and UCB. JC-CW has received research/grant support from AbbVie, Eli Lilly, Gilead Sciences, Janssen, MSD, Novartis, Pfizer Inc, and UCB, is a consultant for Eli Lilly, Novartis, and Pfizer Inc, and is a member of the speakers’ bureaus for Eli Lilly, Janssen, Novartis, and Pfizer Inc. FVdB has received consultancy and/or speaker fees from AbbVie, Celgene, Eli Lilly, Galapagos, Gilead Sciences, GlaxoSmithKline, Janssen, Merck, Novartis, Pfizer Inc, and UCB. MM has received research/grant support from AbbVie and UCB, and has received consultancy fees from AbbVie, Eli Lilly, Janssen, Novartis, Pfizer Inc, and UCB. VS has received consultancy fees from AbbVie, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Celltrion, Eli Lilly, Genentech/Roche, GlaxoSmithKline, Janssen, Merck, Novartis, Pfizer Inc, Regeneron, Samsung, Sandoz, Sanofi, and UCB. LW, DF, JCC, CW, JW, OD, and LF are employees and shareholders of Pfizer Inc.
Figures


References
-
- Bohn R, Cooney M, Deodhar A, et al. . Incidence and prevalence of axial spondyloarthritis: methodologic challenges and gaps in the literature. Clin Exp Rheumatol 2018;36:263–74. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
