Precision Phenotyping of Dilated Cardiomyopathy Using Multidimensional Data
- PMID: 35654493
- PMCID: PMC9168440
- DOI: 10.1016/j.jacc.2022.03.375
Precision Phenotyping of Dilated Cardiomyopathy Using Multidimensional Data
Abstract
Background: Dilated cardiomyopathy (DCM) is a final common manifestation of heterogenous etiologies. Adverse outcomes highlight the need for disease stratification beyond ejection fraction.
Objectives: The purpose of this study was to identify novel, reproducible subphenotypes of DCM using multiparametric data for improved patient stratification.
Methods: Longitudinal, observational UK-derivation (n = 426; median age 54 years; 67% men) and Dutch-validation (n = 239; median age 56 years; 64% men) cohorts of DCM patients (enrolled 2009-2016) with clinical, genetic, cardiovascular magnetic resonance, and proteomic assessments. Machine learning with profile regression identified novel disease subtypes. Penalized multinomial logistic regression was used for validation. Nested Cox models compared novel groupings to conventional risk measures. Primary composite outcome was cardiovascular death, heart failure, or arrhythmia events (median follow-up 4 years).
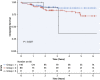
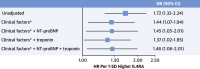
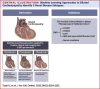
Results: In total, 3 novel DCM subtypes were identified: profibrotic metabolic, mild nonfibrotic, and biventricular impairment. Prognosis differed between subtypes in both the derivation (P < 0.0001) and validation cohorts. The novel profibrotic metabolic subtype had more diabetes, universal myocardial fibrosis, preserved right ventricular function, and elevated creatinine. For clinical application, 5 variables were sufficient for classification (left and right ventricular end-systolic volumes, left atrial volume, myocardial fibrosis, and creatinine). Adding the novel DCM subtype improved the C-statistic from 0.60 to 0.76. Interleukin-4 receptor-alpha was identified as a novel prognostic biomarker in derivation (HR: 3.6; 95% CI: 1.9-6.5; P = 0.00002) and validation cohorts (HR: 1.94; 95% CI: 1.3-2.8; P = 0.00005).
Conclusions: Three reproducible, mechanistically distinct DCM subtypes were identified using widely available clinical and biological data, adding prognostic value to traditional risk models. They may improve patient selection for novel interventions, thereby enabling precision medicine.
Keywords: heart; machine learning; proteomics.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding Support and Author Disclosures This work was supported by the UK Medical Research Council (UT- MR/M003191/1; DOR-MRC: MC-A658-5QEB0), Elliot's Touch, National Institute for Health Research Royal Brompton Biomedical Research Unit, National Institute for Health Research Imperial College Biomedical Research Centre, British Heart Foundation (SP/10/10/28431; SP/17/11/32885; RE/18/4/34215; DOR: RG/19/6/34387), Fondation Leducq (11 CVD-01, 16 CVD-03), Wellcome Trust (107469/Z/15/Z), Rosetrees Trust, Alexander Jansons Foundation, CORDA, and the Society of Cardiovascular Magnetic Resonance. This research was funded in part by the Wellcome Trust. The funders had no input in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Dr Hazebroek has received funding from the Kootstra Talented Post-Doc Fellowship. Dr Ware has served as a consultant for MyoKardia and Foresite Labs. Dr Pennell has served as a consultant for Chiesi; has received research support from Bayer and Siemens; and has received speakers fees from Chiesi and Bayer. Dr Cooper has served as a board member for the Myocarditis Foundation; and has served as a consultant for Kiniksa, CardiolRx, Stromal Therapeutics, and Bristol Myers Squibb. Dr Januzzi is a Trustee of the American College of Cardiology; has received research support from Applied Therapeutics, Innolife, Novartis Pharmaceuticals, and Abbott Diagnostics; has received consulting income from Abbott, Janssen, Novartis, and Roche Diagnostics; and has served on Clinical Endpoint Committees/Data Safety Monitoring Boards for Abbott, AbbVie, Amgen, Bayer, CVRx, Janssen, MyoKardia, and Takeda. Dr Cook is co-founder and a shareholder of Enleofen Bio PTE LTD. Dr Deo has received funding from the National Institutes of Health/National Heart, Lung, and Blood Institute (DP2 HL123228), and One Brave Idea. Prof Heymans has received funding from IMI2-CARDIATEAM (N° 821508), the Netherlands Cardiovascular Research Initiative, an initiative with support of the Dutch Heart Foundation, CVON2016-Early HFPEF, 2015-10, CVONShe-PREDICTS, grant 2017-21, CVON Arena-PRIME, and 2017-18; is supported by FWO G091018N and FWO G0B5930N; has received personal fees for scientific advice to AstraZeneca, Cellprothera, and Merck; and has received an unrestricted research grant from Pfizer. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures



Comment in
-
Dilated Cardiomyopathy: New Distinct Phenotypes or Temporal Phases of Disease?J Am Coll Cardiol. 2022 Jun 7;79(22):2233-2235. doi: 10.1016/j.jacc.2022.04.008. J Am Coll Cardiol. 2022. PMID: 35654494 Free PMC article. No abstract available.
References
-
- Bozkurt B., Colvin M., Cook J., et al. Current diagnostic and treatment strategies for specific dilated cardiomyopathies: a scientific statement from the American Heart Association. Circulation. 2016;134:e579–e646. - PubMed
-
- Pinto Y.M., Elliott P.M., Arbustini E., et al. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: a position statement of the ESC working group on myocardial and pericardial diseases. Eur Heart J. 2016;37:1850–1858. - PubMed
-
- Rapezzi C., Arbustini E., Caforio A.L., et al. Diagnostic work-up in cardiomyopathies: bridging the gap between clinical phenotypes and final diagnosis. A position statement from the ESC Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:1448–1458. - PubMed
-
- Gulati A., Jabbour A., Ismail T.F., et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA. 2013;309:896–908. - PubMed
-
- Merlo M., Cannata A., Pio Loco C., et al. Contemporary survival trends and aetiological characterization in non-ischaemic dilated cardiomyopathy. Eur J Heart Fail. 2020;22:1111–1121. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DP2 HL123228/HL/NHLBI NIH HHS/United States
- MC-A658-5QEB0/MRC_/Medical Research Council/United Kingdom
- MR/M003191/1/MRC_/Medical Research Council/United Kingdom
- RG/19/6/34387/BHF_/British Heart Foundation/United Kingdom
- RE/18/4/34215/BHF_/British Heart Foundation/United Kingdom
- SP/10/10/28431/BHF_/British Heart Foundation/United Kingdom
- NH/17/1/32725/BHF_/British Heart Foundation/United Kingdom
- MC_U120085815/MRC_/Medical Research Council/United Kingdom
- FS/ICRF/21/26019/BHF_/British Heart Foundation/United Kingdom
- MC_UP_1605/13/MRC_/Medical Research Council/United Kingdom
- MC_UP_1102/20/MRC_/Medical Research Council/United Kingdom
- 107469/Z/15/Z/WT_/Wellcome Trust/United Kingdom
- FS/ICRF/22/26039/BHF_/British Heart Foundation/United Kingdom
- SP/17/11/32885/BHF_/British Heart Foundation/United Kingdom
LinkOut - more resources
Full Text Sources
Medical

