Icatibant use in Brazilian patients with hereditary angioedema (HAE) type 1 or 2 and HAE with normal C1-INH levels: findings from the Icatibant Outcome Survey Registry Study
- PMID: 35654647
- PMCID: PMC9263662
- DOI: 10.1016/j.abd.2021.09.009
Icatibant use in Brazilian patients with hereditary angioedema (HAE) type 1 or 2 and HAE with normal C1-INH levels: findings from the Icatibant Outcome Survey Registry Study
Abstract
Background: Hereditary angioedema can be caused by C1-Inhibitor (C1-INH) deficiency and/or dysfunction (HAE-1/2) or can occur in patients with normal C1-INH (HAE nC1-INH).
Methods: The Icatibant Outcome Survey (IOS; NCT01034969) registry monitors the safety and effectiveness of icatibant for treating acute angioedema.
Objective: Present findings from Brazilian patients with HAE-1/2 and HAE nC1-INH participating in IOS.
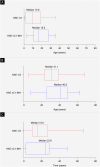
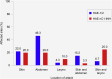
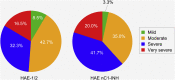
Results: 42 patients were enrolled (HAE-1/2, n = 26; HAE nC1-INH, n = 16). Median age at symptom onset was significantly lower with HAE-1/2 vs. HAE nC1-INH (10.0 vs. 16.5y, respectively; p = 0.0105), whereas median age at diagnosis (31.1 vs. 40.9y; p = 0.1276) and the median time between symptom onset and diagnosis (15.0 vs. 23.8y; p = 0.6680) were numerically lower vs. HAE nC1-INH, respectively. One icatibant dose was used for > 95% of HAE attacks. Median (range) time-to-event outcomes were shorter for patients with HAE nC1-INH vs. HAE-1/2, including time to first administration (0.5 [0-96.0] vs. 1.0 [0-94.0]h, respectively), time from first administration to complete resolution (1.0 [0-88.0] vs. 5.5 [0-96.0]h, respectively), and total attack duration (7.0 [0.3-99.0] vs. 18.5 [0.1-100.0]h, respectively). Mean (SD) time from attack onset to resolution was significantly shorter for patients with HAE nC1-INH vs. HAE-1/2 (9.8 [18.7] vs. 19.6 [24.0]h, respectively; p = 0.0174). 83 adverse events (AEs) in 42 patients were reported; most were mild (66.3%) or moderate (13.3%) and non-serious (75.9%). The most common icatibant-related AE was injection site erythema (HAE-1/2, 34.6%; HAE nC1-INH, 18.8%).
Study limitations: This was an observational study without a treatment comparator and that relied on patient recall.
Conclusions: Findings demonstrate effectiveness and tolerability of icatibant in Brazilian HAE patients.
Keywords: Bradykinin; Bradykinin receptor; Brazil; Hereditary angioedema.
Copyright © 2022. Published by Elsevier España, S.L.U.
Figures


References
-
- Gill P., Betschel S.D. The clinical evaluation of angioedema. Immunol Allergy Clin North Am. 2017;37:449–466. - PubMed
-
- Bork K., Hardt J., Witzke G. Fatal laryngeal attacks and mortality in hereditary angioedema due to C1-INH deficiency. J Allergy Clin Immunol. 2012;130:692–697. - PubMed
-
- Craig T., Busse P., Gower R.G., Johnston D.T., Kashkin J.M., Li H.H., et al. Long-term prophylaxis therapy in patients with hereditary angioedema with C1 inhibitor deficiency. Ann Allergy Asthma Immunol. 2018;121:673–679. - PubMed
-
- Granero-Molina J., Sánchez-Hernández F., Fernández-Sola C., Jiménez-Lasserrotte M.D.M., Antequera-Raynal L.H., Hernández-Padilla J.M. The diagnosis of hereditary angioedema: family caregivers’ experiences. Clin Nurs Res. 2020;29:117–126. - PubMed
-
- Banerji A. The burden of illness in patients with hereditary angioedema. Ann Allergy Asthma Immunol. 2013;111:329–336. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

