A role for microfluidic systems in precision medicine
- PMID: 35654785
- PMCID: PMC9163169
- DOI: 10.1038/s41467-022-30384-7
A role for microfluidic systems in precision medicine
Abstract
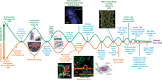
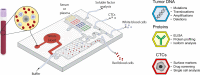
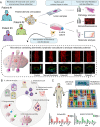
Precision oncology continues to challenge the "one-size-fits-all" dogma. Under the precision oncology banner, cancer patients are screened for molecular tumor alterations that predict treatment response, ideally leading to optimal treatments. Functional assays that directly evaluate treatment efficacy on the patient's cells offer an alternative and complementary tool to improve the accuracy of precision oncology. Unfortunately, traditional Petri dish-based assays overlook much tumor complexity, limiting their potential as predictive functional biomarkers. Here, we review past applications of microfluidic systems for precision medicine and discuss the present and potential future role of functional microfluidic assays as treatment predictors.
© 2022. The Author(s).
Conflict of interest statement
D.J. Beebe holds equity in Bellbrook Labs LLC, Tasso Inc., Stacks to the Future LLC, Lynx Biosciences LLC, Onexio Biosystems LLC, Turba LLC, Flambeau Diagnostics LLC, and Salus Discovery LLC. D.J.J. Lang holds equity in Salus Discovery LLC. J.M. Lang holds equity in Salus Discovery LLC. Lang financial interests: Pfizer (Advisory Board, Personal), Janssen (Advisory Board, Personal), Gilead (Advisory Board, Personal), 4D Pharma (Advisory Board, Personal), Arvinas (Advisory Board, Personal), Astellas (Advisory Board, Personal), Myovant (Advisory Board, Personal), AstraZeneca (Advisory Board, Personal), SeaGen (Advisory Board, Personal). J.M. Ayuso and M. Virumbrales-Muñoz declare no competing interests.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

