Adaptation to simulated microgravity in Streptococcus mutans
- PMID: 35654802
- PMCID: PMC9163064
- DOI: 10.1038/s41526-022-00205-8
Adaptation to simulated microgravity in Streptococcus mutans
Abstract
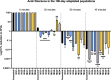
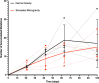
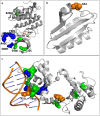
Long-term space missions have shown an increased incidence of oral disease in astronauts' and as a result, are one of the top conditions predicted to impact future missions. Here we set out to evaluate the adaptive response of Streptococcus mutans (etiological agent of dental caries) to simulated microgravity. This organism has been well studied on earth and treatment strategies are more predictable. Despite this, we are unsure how the bacterium will respond to the environmental stressors in space. We used experimental evolution for 100-days in high aspect ratio vessels followed by whole genome resequencing to evaluate this adaptive response. Our data shows that planktonic S. mutans did evolve variants in three genes (pknB, SMU_399 and SMU_1307c) that can be uniquely attributed to simulated microgravity populations. In addition, collection of data at multiple time points showed mutations in three additional genes (SMU_399, ptsH and rex) that were detected earlier in simulated microgravity populations than in the normal gravity controls, many of which are consistent with other studies. Comparison of virulence-related phenotypes between biological replicates from simulated microgravity and control orientation cultures generally showed few changes in antibiotic susceptibility, while acid tolerance and adhesion varied significantly between biological replicates and decreased as compared to the ancestral populations. Most importantly, our data shows the importance of a parallel normal gravity control, sequencing at multiple time points and the use of biological replicates for appropriate analysis of adaptation in simulated microgravity.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests
Figures


References
-
- McPhee, J. C. & Charles, J. B. Human health and performance risks of space exploration missions: evidence reviewed by the NASA human research program (US NASA, Houston, TX, 2009).
-
- Pierson, D. L. Microbial contamination of spacecraft. Gravit. Space Res. 14 (2007). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

