Histone demethylase KDM4C is a functional dependency in JAK2-mutated neoplasms
- PMID: 35654819
- PMCID: PMC9252905
- DOI: 10.1038/s41375-022-01611-3
Histone demethylase KDM4C is a functional dependency in JAK2-mutated neoplasms
Abstract
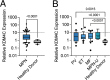
Mutations of the JAK2 gene are frequent aberrations in the aging hematopoietic system and in myeloid neoplasms. While JAK-inhibitors efficiently reduce hyperinflammation induced by the constitutively active mutated JAK2 kinase, the malignant clone and abundance of mutated cells remains rather unaffected. Here, we sought to assess for genetic vulnerabilities of JAK2-mutated clones. We identified lysine-specific demethylase KDM4C as a selective genetic dependency that persists upon JAK-inhibitor treatment. Genetic inactivation of KDM4C in human and murine JAK2-mutated cells resulted in loss of cell competition and reduced proliferation. These findings led to reduced disease penetrance and improved survival in xenograft models of human JAK2-mutated cells. KDM4C deleted cells showed alterations in target histone residue methylation and target gene expression, resulting in induction of cellular senescence. In summary, these data establish KDM4C as a specific dependency and therapeutic target in JAK2-mutated cells that is essential for oncogenic signaling and prevents induction of senescence.
© 2022. The Author(s).
Conflict of interest statement
FHH received fees for consulting services and research funding from Novartis, Celgene, CTI. AH received research support from Novartis, BMS, Pfizer and Incyte. PE received fees for consulting services from Pfizer. The remaining authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

