Noninvasive proteomic biomarkers for alcohol-related liver disease
- PMID: 35654907
- PMCID: PMC9205783
- DOI: 10.1038/s41591-022-01850-y
Noninvasive proteomic biomarkers for alcohol-related liver disease
Abstract
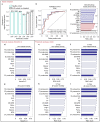
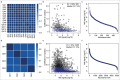
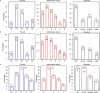
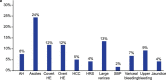
Alcohol-related liver disease (ALD) is a major cause of liver-related death worldwide, yet understanding of the three key pathological features of the disease-fibrosis, inflammation and steatosis-remains incomplete. Here, we present a paired liver-plasma proteomics approach to infer molecular pathophysiology and to explore the diagnostic and prognostic capability of plasma proteomics in 596 individuals (137 controls and 459 individuals with ALD), 360 of whom had biopsy-based histological assessment. We analyzed all plasma samples and 79 liver biopsies using a mass spectrometry (MS)-based proteomics workflow with short gradient times and an enhanced, data-independent acquisition scheme in only 3 weeks of measurement time. In plasma and liver biopsy tissues, metabolic functions were downregulated whereas fibrosis-associated signaling and immune responses were upregulated. Machine learning models identified proteomics biomarker panels that detected significant fibrosis (receiver operating characteristic-area under the curve (ROC-AUC), 0.92, accuracy, 0.82) and mild inflammation (ROC-AUC, 0.87, accuracy, 0.79) more accurately than existing clinical assays (DeLong's test, P < 0.05). These biomarker panels were found to be accurate in prediction of future liver-related events and all-cause mortality, with a Harrell's C-index of 0.90 and 0.79, respectively. An independent validation cohort reproduced the diagnostic model performance, laying the foundation for routine MS-based liver disease testing.
© 2022. The Author(s).
Conflict of interest statement
P.E.G. is a cofounder of OmicEra Diagnostics, a company offering MS-based proteomics analysis, the founding of which was during conduction of the study. M.S. is a former employee at OmicEra Diagnostics. M.M. is currently an indirect investor in Evosep. M.T. has a speaker's fee received from Echosens and Siemens Healthcare. A.K. has served as a speaker for Norgine, Siemens and Nordic Bioscience and has participated in advisory boards for Norgine and Siemens, all outside the submitted work. The remaining authors declare no competing interests.
Figures








Comment in
-
Omics and AI advance biomarker discovery for liver disease.Nat Med. 2022 Jun;28(6):1131-1132. doi: 10.1038/s41591-022-01853-9. Nat Med. 2022. PMID: 35710988 No abstract available.
-
Advancing proteomics and machine learning in the clinic: an editorial on "Noninvasive proteomic biomarkers for alcohol-related liver disease".Hepatobiliary Surg Nutr. 2022 Oct;11(5):762-765. doi: 10.21037/hbsn-22-390. Hepatobiliary Surg Nutr. 2022. PMID: 36268253 Free PMC article. No abstract available.
-
Foreseeing Alcohol-Associated Liver Disease through Proteomic Biomarkers.Clin Chem. 2023 Apr 28;69(5):438-441. doi: 10.1093/clinchem/hvac177. Clin Chem. 2023. PMID: 36609503 Free PMC article. No abstract available.
-
Proteomic Panels for Alcohol-Associated Liver Disease: Accurate, but Different Enough From Existing Clinical Tests?Gastroenterology. 2023 Dec;165(6):1576. doi: 10.1053/j.gastro.2023.09.002. Epub 2023 Sep 6. Gastroenterology. 2023. PMID: 37683704 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

