PragmaTic, prospEctive, randomized, controlled, double-blind, mulTi-centre, multinational study on the safety and efficacy of a 6% HydroxYethyl Starch (HES) solution versus an electrolyte solution in trauma patients: study protocol for the TETHYS study
- PMID: 35655234
- PMCID: PMC9164328
- DOI: 10.1186/s13063-022-06390-x
PragmaTic, prospEctive, randomized, controlled, double-blind, mulTi-centre, multinational study on the safety and efficacy of a 6% HydroxYethyl Starch (HES) solution versus an electrolyte solution in trauma patients: study protocol for the TETHYS study
Abstract
Background: Trauma may be associated with significant to life-threatening blood loss, which in turn may increase the risk of complications and death, particularly in the absence of adequate treatment. Hydroxyethyl starch (HES) solutions are used for volume therapy to treat hypovolemia due to acute blood loss to maintain or re-establish hemodynamic stability with the ultimate goal to avoid organ hypoperfusion and cardiovascular collapse. The current study compares a 6% HES 130 solution (Volulyte 6%) versus an electrolyte solution (Ionolyte) for volume replacement therapy in adult patients with traumatic injuries, as requested by the European Medicines Agency to gain more insights into the safety and efficacy of HES in the setting of trauma care.
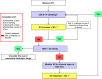
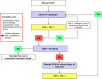
Methods: TETHYS is a pragmatic, prospective, randomized, controlled, double-blind, multicenter, multinational trial performed in two parallel groups. Eligible consenting adults ≥ 18 years, with an estimated blood loss of ≥ 500 ml, and in whom initial surgery is deemed necessary within 24 h after blunt or penetrating trauma, will be randomized to receive intravenous treatment at an individualized dose with either a 6% HES 130, or an electrolyte solution, for a maximum of 24 h or until reaching the maximum daily dose of 30 ml/kg body weight, whatever occurs first. Sample size is estimated as 175 patients per group, 350 patients total (α = 0.025 one-tailed, power 1-β = 0.8). Composite primary endpoint evaluated in an exploratory manner will be 90-day mortality and 90-day renal failure, defined as AKIN stage ≥ 2, RIFLE injury/failure stage, or use of renal replacement therapy (RRT) during the first 3 months. Secondary efficacy and safety endpoints are fluid administration and balance, changes in vital signs and hemodynamic status, changes in laboratory parameters including renal function, coagulation, and inflammation biomarkers, incidence of adverse events during treatment period, hospital, and intensive care unit (ICU) length of stay, fitness for ICU or hospital discharge, and duration of mechanical ventilation and/or RRT.
Discussion: This pragmatic study will increase the evidence on safety and efficacy of 6% HES 130 for treatment of hypovolemia secondary to acute blood loss in trauma patients.
Trial registration: Registered in EudraCT, No.: 2016-002176-27 (21 April 2017) and ClinicalTrials.gov, ID: NCT03338218 (09 November 2017).
Keywords: Acute kidney injury; Blood loss; Colloids; HES; Hydroxyethyl starch; Trauma; Volume therapy.
© 2022. The Author(s).
Conflict of interest statement
Wolfgang Buhre is member of the Steering Committee of the Prodigy Study (sponsored by Medtronic), POSE Study (supported by ESAIC), Designation Study (Supported with a grant from ZonMw), and PI of the AMAZONE study (supported with grants from the Dutch Cancer Society and ESAIC). Ute Brauer (Chief Medical Officer), Tamara Dehnhardt (Director Clinical Operations) and Sonja Schmier (Senior Scientific Manager) are employees of B. Braun Melsungen AG. Martin Westphal (Chief Medical Officer), Dirk Dormann (Vice-President Medical Affairs) and Martin Hernández-González (Director Medical Writing) are employees of Fresenius Kabi Deutschland GmbH.
Figures

References
-
- Chappell D, Jacob M. Fluid and volume therapy in 2013. A balancing act between physiology, evidence, and policy. Notfall Rettungsmed. 2013;16(8):617–624. doi: 10.1007/s10049-013-1805-8. - DOI
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

