Pascal short-pulse plus subthreshold endpoint management laser therapy for diabetic macular edema: the "sandwich technique"
- PMID: 35655248
- PMCID: PMC9161489
- DOI: 10.1186/s40942-022-00381-5
Pascal short-pulse plus subthreshold endpoint management laser therapy for diabetic macular edema: the "sandwich technique"
Abstract
Background: Diabetic macular edema (DME) is the main cause of visual loss in diabetic patients. Despite the use of anti-VEGF therapy as first-line treatment, there are many patients whose response to treatment is poor or transient at best. Sophisticated laser techniques have emerged aiming at low-intensity retinal damage, avoiding excessive heat that causes tissue necrosis and related collateral effects.
Objective: To evaluate the effect of combined sublethal laser modalities from short-pulse duration (SPD) with endpoint management (EpM) subthreshold laser [named the "sandwich technique" (SWiT)] on central subfield thickness (CST) and best-corrected visual acuity (BCVA) in patients with DME.
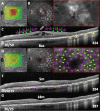
Material and methods: In this consecutive retrospective study, 37 patients (37 eyes) with center-involved (CI) DME were treated with SWiT laser therapy from April 2017 to June 2021. The technique consisted of a mean number of 200 (range number 50-400) SPD laser burns OCT-guided thickened area performed on the juxta- and perifoveal area 500 µm away from the foveal center, overlapping with a mean number of 1000 (range number 800-1200) EpM laser burns focused on 6 mm macular diameter area but saving 300 µm toward the foveal center. All patients underwent ophthalmological evaluations, including BCVA and CST measurement by spectral-domain optical coherence tomography (SD-OCT), before and after SWiT laser therapy. The mean follow-up time was 19.2 months (range 2-60 months).
Results: Thirty-five out of 37 cases showed an improvement in CST and BCVA following treatment. At baseline, mean CST (µm) ± standard error (SE) and mean BCVA (logMAR) ± SE was 456.95 ± 37.00 and 0.71 ± 0.29, respectively. After a mean follow-up of 19.2 months, mean CST (µm) ± SE and BCVA (logMAR) ± SE were 272.09 ± 9.10 (p < 0.0001) and 0.54 ± 0.26 (p = 0.003), respectively. A statistically significant reduction in CST and improvement in BCVA was noted after laser therapy application. The anti-VEGF injection frequency was reduced during the mean 19.2 months of the study period.
Conclusions: The novel "sandwich" laser therapy aid reduced CST and improved BCVA in this retrospective case series. Further prospective studies are warranted.
Keywords: Diabetic macular edema; Macular grid and focal laser; Short-pulse laser; Subthreshold laser.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
LinkOut - more resources
Full Text Sources
Miscellaneous

