Tolerating bad health research: the continuing scandal
- PMID: 35655288
- PMCID: PMC9161194
- DOI: 10.1186/s13063-022-06415-5
Tolerating bad health research: the continuing scandal
Abstract
Background: At the 2015 REWARD/EQUATOR conference on research waste, the late Doug Altman revealed that his only regret about his 1994 BMJ paper 'The scandal of poor medical research' was that he used the word 'poor' rather than 'bad'. But how much research is bad? And what would improve things?
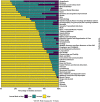
Main text: We focus on randomised trials and look at scale, participants and cost. We randomly selected up to two quantitative intervention reviews published by all clinical Cochrane Review Groups between May 2020 and April 2021. Data including the risk of bias, number of participants, intervention type and country were extracted for all trials included in selected reviews. High risk of bias trials was classed as bad. The cost of high risk of bias trials was estimated using published estimates of trial cost per participant. We identified 96 reviews authored by 546 reviewers from 49 clinical Cochrane Review Groups that included 1659 trials done in 84 countries. Of the 1640 trials providing risk of bias information, 1013 (62%) were high risk of bias (bad), 494 (30%) unclear and 133 (8%) low risk of bias. Bad trials were spread across all clinical areas and all countries. Well over 220,000 participants (or 56% of all participants) were in bad trials. The low estimate of the cost of bad trials was £726 million; our high estimate was over £8 billion. We have five recommendations: trials should be neither funded (1) nor given ethical approval (2) unless they have a statistician and methodologist; trialists should use a risk of bias tool at design (3); more statisticians and methodologists should be trained and supported (4); there should be more funding into applied methodology research and infrastructure (5).
Conclusions: Most randomised trials are bad and most trial participants will be in one. The research community has tolerated this for decades. This has to stop: we need to put rigour and methodology where it belongs - at the centre of our science.
Keywords: Methodologists; Randomised trials; Research waste; Risk of bias; Statisticians.
© 2022. The Author(s).
Conflict of interest statement
ST is an Editor-in-Chief of
Figures
References
-
- Matthews R, Chalmers I, Rothwell P. Douglas G Altman: statistician, researcher, and driving force behind global initiatives to improve the reliability of health research. BMJ. 2018;362:k2588. doi: 10.1136/bmj.k2588. - DOI
-
- Glasziou P, Chalmers IC. Research waste is still a scandal—an essay by Paul Glasziou and Iain Chalmers. MJ. 2018;363:k4645. doi: 10.1136/bmj.k4645. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous