A randomised, crossover, clinical study to assess nicotine pharmacokinetics and subjective effects of the BIDI® stick ENDS compared with combustible cigarettes and a comparator ENDS in adult smokers
- PMID: 35655314
- PMCID: PMC9160848
- DOI: 10.1186/s12954-022-00638-0
A randomised, crossover, clinical study to assess nicotine pharmacokinetics and subjective effects of the BIDI® stick ENDS compared with combustible cigarettes and a comparator ENDS in adult smokers
Abstract
Background: Nicotine pharmacokinetic assessments of electronic nicotine delivery systems (ENDS) are crucial to understand their ability to provide an alternative to cigarette smoking. Subjective effects data also strongly contribute to this understanding. The BIDI® Stick is a disposable ENDS product which contains 59 mg/ml nicotine benzoate salt and various flavours.
Methods: In this study, we assessed nicotine pharmacokinetics and subjective effects of 6 flavour variants of BIDI® Stick ENDS in adult smokers, compared to cigarettes and a comparator ENDS product. During each of eight study visits, 18 volunteer smoker subjects randomly used one of either their usual brand (UB) of cigarette, a BIDI® Stick ENDS, or a comparator ENDS (JUUL 59 mg/ml nicotine with Virginia Tobacco flavour), during both controlled (10 puffs, 30 s apart) and ad libitum (60 min) puffing sessions. Blood samples were collected at various time points and subjective effects questionnaires were administered.
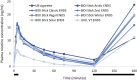
Results: Mean [SD] plasma nicotine Cmax 0-120 was not significantly different between BIDI® Stick ENDS with any flavour (range 15.3 [9.90] ng/ml for BIDI® Stick Winter to 17.6 [9.00] ng/ml for BIDI® Stick Classic) and UB cigarettes (16.2 [9.17] ng/ml). Mean [SD] AUC0-120 (range 569.7 [327.29] to 628.6 [408.99] min*ng/ml for BIDI® Stick ENDS and 747.1 [325.48] min*ng/ml for UB cigarettes) and median Tmax 0-120 (range 5-7 min for all BIDI® Stick ENDS and UB cigarettes) values were also not significantly different between BIDI® Stick ENDS and UB cigarettes, while subjective effects measures were also similar between BIDI® Stick ENDS and UB cigarettes. Mean [SD] plasma nicotine Cmax 0-120, AUC0-120, and median Tmax 0-120 were 6.8 [4.13] ng/ml, 243.6 [179.04] min*ng/ml, and 5 min, respectively, for JUUL ENDS. These values were significantly different compared with those for all BIDI® Stick ENDS and UB cigarettes for both Cmax 0-120 and AUC0-120 but not for Tmax 0-120.
Conclusions: BIDI® Stick ENDS delivered nicotine to users comparably to their UB combustible cigarette and higher than JUUL ENDS, and also elicited similar subjective effects such as satisfaction and relief. Thus, the BIDI® Stick ENDS may be a satisfying alternative to cigarettes among current smokers and may support their transitioning away from cigarette smoking.
Trial registration: ClinicalTrials.gov (identifier number NCT05072925).
Keywords: Cigarette; Electronic nicotine delivery system; Nicotine; Pharmacokinetics; Smoking; Subjective effects.
© 2022. The Author(s).
Conflict of interest statement
IMF is an independent consultant contracted to ENDS and tobacco product manufacturers to provide scientific and regulatory support for clinical and behavioural studies. RGNS is an independent consultant who provides statistical support to ENDS manufacturers. KG is an employee of, and WM is the President of, McKinney Regulatory Science Advisors, LLC who are contracted to provide scientific and regulatory support to ENDS and tobacco product manufacturers.
Figures
Similar articles
-
An Open-Label, Randomized, Controlled, Crossover Study to Assess Nicotine Pharmacokinetics and Subjective Effects of the JUUL System with Three Nicotine Concentrations Relative to Combustible Cigarettes in Adult Smokers.Nicotine Tob Res. 2021 May 24;23(6):947-955. doi: 10.1093/ntr/ntab001. Nicotine Tob Res. 2021. PMID: 33486526 Free PMC article. Clinical Trial.
-
Abuse liability assessment of the JUUL system in four flavors relative to combustible cigarette, nicotine gum and a comparator electronic nicotine delivery system among adult smokers.Drug Alcohol Depend. 2020 Dec 1;217:108395. doi: 10.1016/j.drugalcdep.2020.108395. Epub 2020 Nov 4. Drug Alcohol Depend. 2020. PMID: 33176942
-
Assessment of Abuse Liability and Nicotine Pharmacokinetics of glo Heated Tobacco Products in a Randomized, Crossover Study.Eur J Drug Metab Pharmacokinet. 2024 Nov;49(6):733-750. doi: 10.1007/s13318-024-00921-4. Epub 2024 Oct 25. Eur J Drug Metab Pharmacokinet. 2024. PMID: 39453550 Free PMC article. Clinical Trial.
-
Nicotine delivery and cigarette equivalents from vaping a JUULpod.Tob Control. 2022 Aug;31(e1):e88-e93. doi: 10.1136/tobaccocontrol-2020-056367. Epub 2021 Mar 24. Tob Control. 2022. PMID: 33762429 Free PMC article. Review.
-
Human abuse liability assessment of e-cigarettes: Why, what and how?Drug Test Anal. 2023 Oct;15(10):1211-1221. doi: 10.1002/dta.3251. Epub 2022 Mar 30. Drug Test Anal. 2023. PMID: 35302289 Review.
Cited by
-
Part one: abuse liability of Vuse Solo (G2) electronic nicotine delivery system relative to combustible cigarettes and nicotine gum.Sci Rep. 2022 Dec 21;12(1):22080. doi: 10.1038/s41598-022-26417-2. Sci Rep. 2022. PMID: 36543869 Free PMC article. Clinical Trial.
-
Disposable e-cigarettes and their nicotine delivery, usage pattern, and subjective effects in occasionally smoking adults.Sci Rep. 2025 May 9;15(1):16270. doi: 10.1038/s41598-025-97491-5. Sci Rep. 2025. PMID: 40346191 Free PMC article.
-
The role of sweet/fruit-flavored disposable electronic cigarettes on early nicotine initiation - a systematic review.BMC Public Health. 2025 Feb 17;25(1):643. doi: 10.1186/s12889-025-21897-z. BMC Public Health. 2025. PMID: 39962478 Free PMC article.
-
An observational human laboratory assessment of nicotine delivery, vaping topography and subjective effects of usual brand electronic cigarette use among young adults.Addiction. 2025 Mar;120(3):503-513. doi: 10.1111/add.16531. Epub 2024 May 27. Addiction. 2025. PMID: 38800982 Free PMC article.
-
Nicotine Delivery of a Menthol-Flavored Heat-not-Burn Tobacco Product During Directed Use.Nicotine Tob Res. 2024 Feb 22;26(3):397-401. doi: 10.1093/ntr/ntad119. Nicotine Tob Res. 2024. PMID: 37434562 Free PMC article.
References
-
- World Health Organization. Tobacco; 2021. Available From: https://Www.Who.Int/News-Room/Fact-Sheets/Detail/Tobacco.
-
- Institute Of Medicine . Clearing the smoke - assessing the science base for tobacco harm reduction. Washington: The National Academies Press; 2001. - PubMed
-
- Perfetti T, Rodgman A. The complexity of tobacco and tobacco smoke. Beitr Zur Tab Int. 2011;24:17.
-
- Food and Drug Administration. Harmful and potentially harmful constituents in tobacco products and tobacco smoke; established list. Docket No. FDA–2012–N–0143. Federal Register, 2012.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

