A first-in-human phase 1/2 study of FGF401 and combination of FGF401 with spartalizumab in patients with hepatocellular carcinoma or biomarker-selected solid tumors
- PMID: 35655320
- PMCID: PMC9161616
- DOI: 10.1186/s13046-022-02383-5
A first-in-human phase 1/2 study of FGF401 and combination of FGF401 with spartalizumab in patients with hepatocellular carcinoma or biomarker-selected solid tumors
Abstract
Background: Deregulation of FGF19-FGFR4 signaling is found in several cancers, including hepatocellular carcinoma (HCC), nominating it for therapeutic targeting. FGF401 is a potent, selective FGFR4 inhibitor with antitumor activity in preclinical models. This study was designed to determine the recommended phase 2 dose (RP2D), characterize PK/PD, and evaluate the safety and efficacy of FGF401 alone and combined with the anti-PD-1 antibody, spartalizumab.
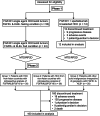
Methods: Patients with HCC or other FGFR4/KLB expressing tumors were enrolled. Dose-escalation was guided by a Bayesian model. Phase 2 dose-expansion enrolled patients with HCC from Asian countries (group1), non-Asian countries (group2), and patients with other solid tumors expressing FGFR4 and KLB (group3). FGF401 and spartalizumab combination was evaluated in patients with HCC.
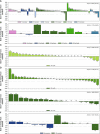
Results: Seventy-four patients were treated in the phase I with single-agent FGF401 at 50 to 150 mg. FGF401 displayed favorable PK characteristics and no food effect when dosed with low-fat meals. The RP2D was established as 120 mg qd. Six of 70 patients experienced grade 3 dose-limiting toxicities: increase in transaminases (n = 4) or blood bilirubin (n = 2). In phase 2, 30 patients in group 1, 36 in group 2, and 20 in group 3 received FGF401. In total, 8 patients experienced objective responses (1 CR, 7 PR; 4 each in phase I and phase II, respectively). Frequent adverse events (AEs) were diarrhea (73.8%), increased AST (47.5%), and ALT (43.8%). Increase in levels of C4, total bile acid, and circulating FGF19, confirmed effective FGFR4 inhibition. Twelve patients received FGF401 plus spartalizumab. RP2D was established as FGF401 120 mg qd and spartalizumab 300 mg Q3W; 2 patients reported PR.
Conclusions: At biologically active doses, FGF401 alone or combined with spartalizumab was safe in patients with FGFR4/KLB-positive tumors including HCC. Preliminary clinical efficacy was observed. Further clinical evaluation of FGF401 using a refined biomarker strategy is warranted.
Trial registration: NCT02325739 .
Keywords: FGF19; FGFR4; Hepatocellular carcinoma; Immune checkpoint inhibitors; KLB; PD-1; PD-L1; Phase 1.
© 2022. The Author(s).
Conflict of interest statement
S. L. Chan: Research fund from Bayer, Eisai, Ipsen, MSD, Sirtex; advisors for AstraZeneca, BMS, Eisai, Novartis, MSD.
M. Schuler: Consultant (compensated) for AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Novartis, Roche, Takeda; honoraria for CME presentations from Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, MSD, Novartis; research funding to institution from AstraZeneca, Boehringer Ingelheim, Bristol Myers-Squibb, Novartis.
Y.-K Kang: Consulting fee from ALX Oncology, Amgen, Blueprint, BMS, Daehwa, MacroGenics, Merck, Novartis, Roche, Surface Oncology, Zymeworks.
C.-J Yen: No conflicts of interest to declare.
J. Edeline: Consultancy/honoraria from AstraZeneca, Bayer, BMS, Boston Scientific, Eisai, Ipsen, MSD, Roche; grant funding from BeiGene, BMS, Boston Scientific.
S.P. Choo: Consultancy/honoraria from Bayer, BMS, Eisai, Ipsen, MSD, Roche; Speaker fees from BMS, Eisai, Eli Lilly, Roche, Sirtex; grant funding from BMS, Sirtex.
C.-C. Lin: Consulting fee from AbbVie, Bayer, Blueprint Medicines, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, Novartis; honoraria from Eli Lilly, Novartis, Roche; support for attending meeting or travel from BeiGene, Daiichi Sankyo, and Eli Lilly.
T. Okusaka: Grant and honoraria from Novartis.
K.-H. Weiss: grants from Alexion, Novartis, Orphalan, Univar; consulting fee from Alexion, Bayer, Chiesi, Orphalan, Pfizer, Ultragenyx, Univar, Vivet therapeutics; honoraria from Falk; support for attending meeting from AbbVie, Bayer, Gilead.
T. Macarulla: Consultancy/advisory role with Amgen, Baxter, Celgene, Incyte, Q&D Therapeutics, Servier, Shire; research funding from AstraZeneca, BeiGene, Celgene.
S. Cattan: grants, consulting fee and honoraria from AstraZeneca, Bayer, Ipsen, Roche; payment for expert testimony and support for travel from AstraZeneca, Ipsen, Roche; participation in advisory board for Roche.
J.-F. Blanc: honoraria from Bayer, Ipsen, Roche; support for attending meeting/travel from Bayer and Ipsen; participation in safety/advisory board for AstraZeneca, Bayer, BMS, Eisai, Ipsen, Eli Lilly, MSD, Roche.
K.-H Lee: honoraria from AstraZeneca and Roche; participated in advisory board for Surface Oncology.
M. Maur: No conflicts of interest to declare.
S. Pant: Consulting or advisory role for 4D Pharma, Ipsen, Xencor, Zymeworks; research funding from 4D Pharma, Arcus Biosciences, ArQule, Astellas Pharma, Boehringer Ingelheim, Bristol-Myers Squibb, Elicio Therapeutics, Five Prime Therapeutics, GlaxoSmithKline, Ipsen, Janssen, Lilly, Mirati Therapeutics, NGM Biopharmaceuticals, Novartis, Onco Response, Purple Biotech, RedHill Biopharma, Rgenix, Sanofi/Aventis, Xencor.
M. Kudo: Consulting fee from BMS, Eisai, MSD, Ono Pharmaceuticals, Roche; honoraria from Bayer, BMS, Chugai, EA Pharma, Eisai, Eli Lilly, MSD; Research funding from AbbVie, Chugai, Eisai, EA Pharma, Gilead Sciences, Ono Pharmaceutical Co, Otsuka, Sumitomo Dainippon Pharma, Taiho, Takeda.
E. Assenat: No conflicts of interest to declare.
A.X. Zhu: Consulting fee from Bayer, BMS, Eisai, Eli Lilly, Exelixis, Roche, Sanofi.
T. Yau: Consulting or advisory role and honoraria from AbbVie, AstraZeneca, Bayer, Bristol Myers-Squibb, Eisai, Eli Lilly, EMD Serono, Exelixis, H3 Biomedicine, Ipsen, MSD, New B Innovation, Novartis, OrigiMed, Pfizer, SillaJen, Sirtex, Taiho.
H.Y. Lim: Participation on data safety monitoring committee or advisory board from Bayer, BMS, Eisai, Merck Serono and Roche.
J. Bruix: Consultancy from AbbVie, Adaptimmune, ArQule, Astra-Medimmune, Basilea, Bayer Shering Pharma, Bio-Alliance, BMS, BTG, Eisai, Gilead, Incyte, Ipsen, Kowa, Lilly, MSD, Nerviano, Novartis, Polaris, Quirem, Roche, Sirtex, Sanofi, Terumo; Research grants from Bayer, Ipsen; Educational grants from Bayer; Paid conferences from Bayer, BTG and Ipsen; Paid talks for Bayer Shering Pharma, BTG Biocompatibles, Eisai, Ipsen Sirtex, Terumo.
A. Geier: Advisor and Steering Committee member for AbbVie, Alexion, Bayer, BMS, Eisai, Gilead, Intercept, Ipsen, Novartis, Pfizer, Roche, Sanofi-Aventis, Sequana; Speaker fees from AbbVie, Alexion, BMS, CSL Behring, Falk, Gilead, Intercept, Merz, Novartis, Roche, Sequana; Research support from Intercept and Falk (NAFLD CSG), Novartis.
C. Guillén-Ponce: Registration for continuing education courses and congresses by Astra Zeneca, Merck Serono and Sanofi.
A. Fasolo: No conflicts of interest to declare.
R.S. Finn: Grants from Adaptimmune, Bayer, BMS, Eisai, Eli Lilly, Merck, Pfizer, Roche/Genentech; consulting fee from Adaptimmune, AstraZeneca, Bayer, BMS, CStone, Eisai, Eli Lilly, Merck, Pfizer, Roche/ Genentech.
J. Fan: No conflicts of interest to declare.
A. Vogel: consulting fee from Amgen, AstraZeneca, Bayer, BMS, BTG, EISAI, GSK, Incyte, Ipsen, Lilly, Merck, PierreFabre, MSD, Novartis, Roche, Sanofi, Servier, Sirtex, Terumo,; honoraria from Amgen, AstraZeneca, Bayer, BMS, BTG, EISAI, GSK, Incyte, Ipsen, Lilly, Merck, MSD, Novartis, PierreFabre, Roche, Sanofi, Servier, Sirtex, Terumo.
S. Qin: No conflicts of interest to declare.
M. Riester: Novartis employee.
V. Katsanou: Novartis employee.
M. Chaudhari: worked for Novartis during employment at IQVIA.
T. Kakizume: Novartis employee at the time of study and manuscript writing; currently working at Takeda.
Yi Gu: Novartis employee and stock holder.
D. Graus Porta: Novartis employee and stockholder at the time of study conduct and manuscript finalization.
A. Myers: Novartis employee.
J-P. Delord: Consulting fee from BMS, MSD, Roche; honoraria from Roche; participation on data safety monitoring board or advisory board for BMS, MSD, Novartis, Transgene.
Figures

References
-
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D. Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

