The Impact of RNA-DNA Hybrids on Genome Integrity in Bacteria
- PMID: 35655343
- PMCID: PMC9527769
- DOI: 10.1146/annurev-micro-102521-014450
The Impact of RNA-DNA Hybrids on Genome Integrity in Bacteria
Abstract
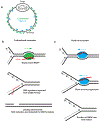
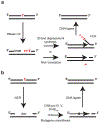
During the essential processes of DNA replication and transcription, RNA-DNA hybrid intermediates are formed that pose significant risks to genome integrity when left unresolved. To manage RNA-DNA hybrids, all cells rely on RNase H family enzymes that specifically cleave the RNA portion of the many different types of hybrids that form in vivo. Recent experimental advances have provided new insight into how RNA-DNA hybrids form and the consequences to genome integrity that ensue when persistent hybrids remain unresolved. Here we review the types of RNA-DNA hybrids, including R-loops, RNA primers, and ribonucleotide misincorporations, that form during DNA replication and transcription and discuss how each type of hybrid can contribute to genome instability in bacteria. Further, we discuss how bacterial RNase HI, HII, and HIII and bacterial FEN enzymes contribute to genome maintenance through the resolution of hybrids.
Keywords: RNA-DNA hybrids; RNase HI; RNase HII; RNase HIII; genome instability; mutation rate.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

