New Sesquiterpenoids From Plant-Associated Irpex lacteus
- PMID: 35655702
- PMCID: PMC9152251
- DOI: 10.3389/fchem.2022.905108
New Sesquiterpenoids From Plant-Associated Irpex lacteus
Erratum in
-
Corrigendum: New Sesquiterpenoids From Plant-Associated Irpex lacteus.Front Chem. 2022 Jun 24;10:946835. doi: 10.3389/fchem.2022.946835. eCollection 2022. Front Chem. 2022. PMID: 35815215 Free PMC article.
Abstract
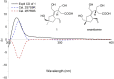
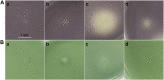
Bacteria produce a large number of virulence factors through the quorum sensing (QS) mechanism. Inhibiting such QS system of the pathogens without disturbing their growth is a potential strategy to control multi-drug-resistant pathogens. To accomplish this, two new tremulane-type sesquiterpenoids, irpexolaceus H (1) and I (2), along with two known furan compounds, irpexlacte B (3) and C (4), were isolated from Orychophragmus violaceus (L.) OE Schulz endophytic fungus Irpex lacteus (Fr.) Fr. Their structures were elucidated by detailed spectroscopic data (NMR, HRESIMS, IR, and UV), single-crystal X-ray diffraction, and electronic circular dichroism (ECD) analysis. Furthermore, those compounds were evaluated for anti-quorum sensing (anti-QS) activity, and compound 3 was found contributing to the potential QS inhibitory activity.
Keywords: Irpex lacteus (Fr.) Fr; Orychophragmus violaceus (L.) O.E. Schulz; furan; quorum sensing; sesquiterpenoids.
Copyright © 2022 Luo, Jiang, Huang and Jia.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
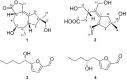

 ), HMBC (
), HMBC ( ), and NOESY (
), and NOESY ( ) correlations of 1 and 2.
) correlations of 1 and 2.
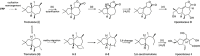
References
-
- Ayer W. A., Browne L. M. (1981). Terpenoid Metabolites of Mushrooms and Related Basidiomycetes . Tetrahedron 37 (12), 2197–2248. 10.1016/S0040-4020(01)97979-7 - DOI
-
- Ayer W. A., Cruz E. R. (1993). The Tremulanes, a New Group of Sesquiterpenes from the aspen Rotting Fungus Phellinus Tremulae . J. Org. Chem. 58 (26), 7529–7534. 10.1021/jo00078a035 - DOI
LinkOut - more resources
Full Text Sources

