Neuroimaging at 7 Tesla: a pictorial narrative review
- PMID: 35655840
- PMCID: PMC9131333
- DOI: 10.21037/qims-21-969
Neuroimaging at 7 Tesla: a pictorial narrative review
Abstract
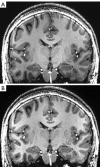
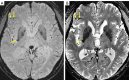
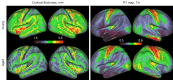
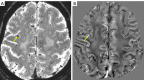
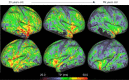
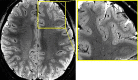
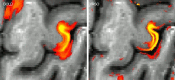
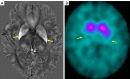
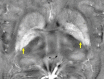
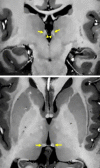
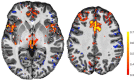
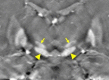
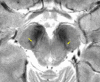
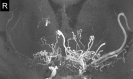
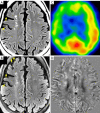
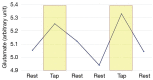
Neuroimaging using the 7-Tesla (7T) human magnetic resonance (MR) system is rapidly gaining popularity after being approved for clinical use in the European Union and the USA. This trend is the same for functional MR imaging (MRI). The primary advantages of 7T over lower magnetic fields are its higher signal-to-noise and contrast-to-noise ratios, which provide high-resolution acquisitions and better contrast, making it easier to detect lesions and structural changes in brain disorders. Another advantage is the capability to measure a greater number of neurochemicals by virtue of the increased spectral resolution. Many structural and functional studies using 7T have been conducted to visualize details in the white matter and layers of the cortex and hippocampus, the subnucleus or regions of the putamen, the globus pallidus, thalamus and substantia nigra, and in small structures, such as the subthalamic nucleus, habenula, perforating arteries, and the perivascular space, that are difficult to observe at lower magnetic field strengths. The target disorders for 7T neuroimaging range from tumoral diseases to vascular, neurodegenerative, and psychiatric disorders, including Alzheimer's disease, Parkinson's disease, multiple sclerosis, epilepsy, major depressive disorder, and schizophrenia. MR spectroscopy has also been used for research because of its increased chemical shift that separates overlapping peaks and resolves neurochemicals more effectively at 7T than a lower magnetic field. This paper presents a narrative review of these topics and an illustrative presentation of images obtained at 7T. We expect 7T neuroimaging to provide a new imaging biomarker of various brain disorders.
Keywords: 7 Tesla (7T); MP2RAGE; functional magnetic resonance imaging (fMRI); magnetic resonance spectroscopy (MRS); susceptibility.
2022 Quantitative Imaging in Medicine and Surgery. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-21-969/coif). TO receives a research grant from Siemens Healthcare K.K., Japan and the JSPS (21H03806). TO serves as an unpaid editorial board member for Quantitative Imaging in Medicine and Surgery. NS and TM receive grants from AMED (21dm0307003h0004 and 21dm0307102h0003, respectively). The other authors have no conflicts of interest to declare.
Figures