An unprecedented azobenzene-based organic single-component ferroelectric
- PMID: 35655879
- PMCID: PMC9067575
- DOI: 10.1039/d2sc00689h
An unprecedented azobenzene-based organic single-component ferroelectric
Abstract
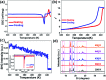
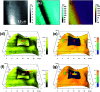
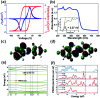
Organic single-component ferroelectrics, as an important class of metal-free ferroelectrics, are highly desirable because of their easy processing, mechanical flexibility, and biocompatibility. However, although nearly 50 years have passed since the discovery of photochromism in azobenzene-doped cholesteric liquid crystals, ferroelectricity has never been found in azobenzene-based crystals. Here, we use an amino group to substitute a fluorine atom of 2,2',4,4',6,6'-hexafluoroazobenzene, which successfully introduces ferroelectricity into 2-amino-2',4,4',6,6'-pentafluoroazobenzene (APFA). APFA shows an extremely high Curie temperature (T c) of 443 K, which is outstanding among single-component ferroelectrics. It also exhibits an indirect optical band gap of 2.27 eV as well as photoisomerization behavior between the trans-form and the cis-form triggered by pedal motion. To our knowledge, APFA is the first azobenzene-based ferroelectric crystal. This work opens an avenue to design excellent single-component ferroelectrics and will inspire the exploration of azobenzene-based ferroelectrics for promising applications in biofriendly ferroelectric devices.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

References
-
- Valasek J. Phys. Rev. 1921;17:475–481. doi: 10.1103/PhysRev.17.475. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

