A Deep Look at the Vaginal Environment During Pregnancy and Puerperium
- PMID: 35656029
- PMCID: PMC9152327
- DOI: 10.3389/fcimb.2022.838405
A Deep Look at the Vaginal Environment During Pregnancy and Puerperium
Abstract
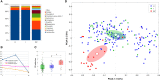
A deep comprehension of the vaginal ecosystem may hold promise for unraveling the pathophysiology of pregnancy and may provide novel biomarkers to identify subjects at risk of maternal-fetal complications. In this prospective study, we assessed the characteristics of the vaginal environment in a cohort of pregnant women throughout their different gestational ages and puerperium. Both the vaginal bacterial composition and the vaginal metabolic profiles were analyzed. A total of 63 Caucasian women with a successful pregnancy and 9 subjects who had a first trimester miscarriage were enrolled. For the study, obstetric examinations were scheduled along the three trimester phases (9-13, 20-24, 32-34 gestation weeks) and puerperium (40-55 days after delivery). Two vaginal swabs were collected at each time point, to assess the vaginal microbiome profiling (by Nugent score and 16S rRNA gene sequencing) and the vaginal metabolic composition (1H-NMR spectroscopy). During pregnancy, the vaginal microbiome underwent marked changes, with a significant decrease in overall diversity, and increased stability. Over time, we found a significant increase of Lactobacillus and a decrease of several genera related to bacterial vaginosis (BV), such as Prevotella, Atopobium and Sneathia. It is worth noting that the levels of Bifidobacterium spp. tended to decrease at the end of pregnancy. At the puerperium, a significantly lower content of Lactobacillus and higher levels of Gardnerella, Prevotella, Atopobium, and Streptococcus were observed. Women receiving an intrapartum antibiotic prophylaxis for Group B Streptococcus (GBS) were characterized by a vaginal abundance of Prevotella compared to untreated women. Analysis of bacterial relative abundances highlighted an increased abundance of Fusobacterium in women suffering a first trimester abortion, at all taxonomic levels. Lactobacillus abundance was strongly correlated with higher levels of lactate, sarcosine, and many amino acids (i.e., isoleucine, leucine, phenylalanine, valine, threonine, tryptophan). Conversely, BV-associated genera, such as Gardnerella, Atopobium, and Sneathia, were related to amines (e.g., putrescine, methylamine), formate, acetate, alcohols, and short-chain fatty-acids (i.e., butyrate, propionate).
Keywords: miscarriage; pregnancy; puerperium; vaginal metabolome; vaginal microbiome; women’s health.
Copyright © 2022 Severgnini, Morselli, Camboni, Ceccarani, Laghi, Zagonari, Patuelli, Pedna, Sambri, Foschi, Consolandi and Marangoni.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

