Blocking C3d+/GFAP+ A1 Astrocyte Conversion with Semaglutide Attenuates Blood-Brain Barrier Disruption in Mice after Ischemic Stroke
- PMID: 35656116
- PMCID: PMC9116904
- DOI: 10.14336/AD.2021.1029
Blocking C3d+/GFAP+ A1 Astrocyte Conversion with Semaglutide Attenuates Blood-Brain Barrier Disruption in Mice after Ischemic Stroke
Abstract
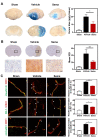
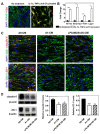
Astrocytes play an essential role in the modulation of blood-brain barrier function. Neurological diseases induce the transformation of astrocytes into a neurotoxic A1 phenotype, exacerbating brain injury. However, the effect of A1 astrocytes on the BBB dysfunction after stroke is unknown. Adult male ICR mice (n=97) were subjected to 90-minute transient middle cerebral artery occlusion (tMCAO). Immunohistochemical staining of A1 (C3d) and A2 (S100A10) was performed to characterize phenotypic changes in astrocytes over time after tMCAO. The glucagon-like peptide-1 receptor agonist semaglutide was intraperitoneally injected into mice to inhibit A1 astrocytes. Infarct volume, atrophy volume, neurobehavioral outcomes, and BBB permeability were evaluated. RNA-seq was adopted to explore the potential targets and signaling pathways of A1 astrocyte-induced BBB dysfunction. Astrocytic C3d expression was increased, while expression of S100A10 was decreased in the first two weeks after tMCAO, reflecting a shift in the astrocytic phenotype. Semaglutide treatment reduced the expression of CD16/32 in microglia and C3d in astrocytes after ischemic stroke (p<0.05). Ischemia-induced brain infarct volume, atrophy volume and neuroinflammation were reduced in the semaglutide-treated mice, and neurobehavioral outcomes were improved compared to control mice (p<0.05). We further demonstrated that semaglutide treatment reduced the gap formation of tight junction proteins ZO-1, claudin-5 and occludin, as well as IgG leakage three days following tMCAO (p<0.05). In vitro experiments revealed that A1 astrocyte-conditioned medium disrupted BBB integrity. RNA-seq showed that A1 astrocytes were enriched in inflammatory factors and chemokines and significantly modulated the TNF and chemokine signaling pathways, which are closely related to barrier damage. We concluded that astrocytes undergo a phenotypic shift over time after ischemic stroke. C3d+/GFAP+ astrocytes aggravate BBB disruption, suggesting that inhibiting C3d+/GFAP+ astrocyte formation represents a novel strategy for the treatment of ischemic stroke.
Keywords: A1 astrocyte; blood-brain barrier; ischemia; neuroinflammation; stroke.
Copyright: © 2022 Zhang et al.
Conflict of interest statement
Competing interests No competing interest.
Figures





References
LinkOut - more resources
Full Text Sources
Miscellaneous
