Investigation of Eutectic Mixtures of Fatty Acids as a Novel Construct for Temperature-Responsive Drug Delivery
- PMID: 35656165
- PMCID: PMC9151329
- DOI: 10.2147/IJN.S359664
Investigation of Eutectic Mixtures of Fatty Acids as a Novel Construct for Temperature-Responsive Drug Delivery
Retraction in
-
Investigation of Eutectic Mixtures of Fatty Acids as a Novel Construct for Temperature-Responsive Drug Delivery [Retraction].Int J Nanomedicine. 2023 Nov 7;18:6409-6410. doi: 10.2147/IJN.S448407. eCollection 2023. Int J Nanomedicine. 2023. PMID: 37954455 Free PMC article.
Abstract
Background: Most of the traditional nanocarriers of cancer therapeutic moieties present dose-related toxicities due to the uptake of chemotherapeutic agents in normal body cells. The severe life-threatening effects of systemic chemotherapy are well documented. Doxorubicin, DOX is the most effective antineoplastic agent but with the least specific action that is responsible for severe cardiotoxicity and myelosuppression that necessitates careful monitoring while administering. Stimuli-sensitive/intelligent drug delivery systems, specifically those utilizing temperature as an external stimulus to activate the release of encapsulated drugs, have become a subject of recent research. Thus, it would be ideal to have a nanocarrier comprising safe excipients and controllable drug release capacity to deliver the drug at a particular site to minimize unwanted and toxic effects of chemotherapeutics. We have developed a simple temperature-responsive nanocarrier based on eutectic mixture of fatty acids. This study aimed to develop, physicochemically characterize and investigate the biological safety of eutectic mixture of fatty acids as a novel construct for temperature-responsive drug release potential.
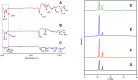
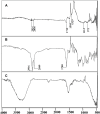
Methods: We have developed phase change material, PCM, based on a series of eutectic mixtures of fatty acids due to their unique and attractive physicochemical characteristics such as safety, stability, cost-effectiveness, and ease of availability. The reversible solid-liquid phase transition of PCM is responsible to hold firm or actively release the encapsulated drug. The eutectic mixtures of fatty acids (stearic acid and myristic acid) along with liquid lipid (oleic acid) were prepared to exhibit a tunable thermoresponsive platform. Doxorubicin-loaded lipid nanocarriers were successfully developed with combined hot melt encapsulation (HME) and sonication method and characterized to achieve enhanced permeability and retention (EPR) effect-based solid tumor targeting in response to exogenous temperature stimulus. The cytotoxicity against melanoma cell lines and in vivo safety studies in albino rats was also carried out.
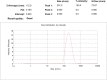
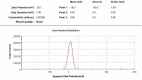
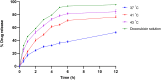
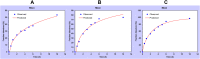
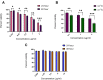
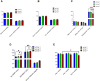
Results: Doxorubicin-loaded lipid nanocarriers have a narrow size distribution (94.59-219.3 nm), and a PDI (0.160-0.479) as demonstrated by photon correlation microscopy and excellent colloidal stability (Z.P value: -22.7 to -32.0) was developed. Transmission electron microscopy revealed their spherical morphology and characteristics of a monodispersed system. A biphasic drug release pattern with a triggered drug release at 41°C and 43°C and a sustained drug release was observed at 37°C. The thermoresponsive cytotoxic potential was demonstrated in B16F10 cancer cell lines. Hemolysis assay and acute toxicity studies with drug-free and doxorubicin lipid nanocarrier formulations provided evidence for their non-toxic nature.
Conclusion: We have successfully developed a temperature-responsive tunable platform with excellent biocompatibility and intelligent drug release potential. The formulation components being from natural sources present superior characteristics in terms of cost, compatibility with normal body cells, and adaptability to preparation methods. The reported preparation method is adapted to avoid complex chemical processes and the use of organic solvents. The lipid nanocarriers with tunable thermoresponsive characteristics are promising biocompatible drug delivery systems for improved localized delivery of chemotherapeutic agents.
Keywords: acute toxicity studies; doxorubicin; eutectic mixtures; fatty acids; hemolysis assay; lipid nanocarriers; nanostructured lipid carriers; phase change materials; thermoresponsive.
© 2022 Parveen et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures



References
-
- World Health Organization. Key facts of cancer; 2018. Available from: https://www.who.int/news-room/fact-sheets/detail/cancer. Accessed December 18, 2019.
-
- World Health Organization. Cancer; 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/cancer. Accessed June 30, 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

