Persistence and Adherence to Biologics in Patients with Psoriasis in Taiwan: A New Biologics User Cohort Study
- PMID: 35656306
- PMCID: PMC9152324
- DOI: 10.3389/fphar.2022.880985
Persistence and Adherence to Biologics in Patients with Psoriasis in Taiwan: A New Biologics User Cohort Study
Abstract
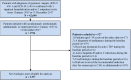
Background: Biologics are used to treat moderate-to-severe psoriasis, and persistence to biologics may reflect clinical effectiveness. Limited information describing how biologics are used in patients with moderate-to-severe psoriasis in Asian countries is available. We conducted a population-based, retrospective, new user cohort study using the National Health Insurance Research Database (NHIRD) in Taiwan to assess treatment persistence and adherence to biologics. Methods: Adults with a diagnosis of psoriasis between 01 January 2015 and 31 December 2017 were identified in the NHIRD (ICD-9-CM 696.1; ICD-10 L40.0). New users were patients who initiated treatment with etanercept, adalimumab, ustekinumab or secukinumab between 01 January 2015 and 31 December 2017. All eligible patients were followed until 31 December 2018, death or disenrollment. Kaplan-Meier analysis was conducted to estimate persistence of treatment for index biologics. A Cox-proportional hazard regression model was used to compare risks of biologic discontinuation between biologic groups. Adjustments for potential confounding factors (age, gender and Charlson comorbidity index score) were made in the Cox model. Results: There were 1,397 new biologic users with psoriasis during the study period. The ratio men:women was approximately 4:1. Mean age of patients ranged from 44.6 to 47.7 years across exposure groups. The 1-year/2-years persistence rates were 94.2%/84.9% for ustekinumab, 96.2%/not calculated (due to too few patients at year 2) for secukinumab, 66.0%/29.9% for etanercept, and 59.8%/40.3% for adalimumab. The risk of discontinuation was significantly lower in patients initiating ustekinumab compared with adalimumab (hazard ratio adjusted for age, sex and co-morbidities 0.289, 95%CI 0.247-0.339, p < 0.0001). Drug survival was significantly higher for ustekinumab compared with adalimumab and etanercept (log-rank test p < 0.0001). The proportions of patients with 1-year/2-years medication possession ratios of ≥80% were 95.3%/92.0% for ustekinumab, 98.1%/not calculated for secukinumab, 89.4%/83.1% for etanercept, and 70.8%/59.4% for adalimumab. Limitations: Clinical improvement and response to treatment data were not available. Conclusion: There was relatively high persistence amongst biologic users with psoriasis in Taiwan. There is a trend towards greater persistence of ustekinumab compared to other biologics, the magnitude of which depends on the treatment gap used for its calculation. This study provides real-world evidence that may facilitate optimal treatment choice.
Keywords: adherence; cohort study; persistence; psoriasis; ustekinumab.
Copyright © 2022 Huang, Tang, Goh, Chang, Qiu, Yang, Saadoun, Chang and Liu.
Conflict of interest statement
CG, HQ and YL are employees of Janssen Research & Development LLC. Y-WY is an employee of Janssen Pharmaceuticals. HQ and YL hold stock in Johnson & Johnson. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Drug Survival Associated With Effectiveness and Safety of Treatment With Guselkumab, Ixekizumab, Secukinumab, Ustekinumab, and Adalimumab in Patients With Psoriasis.JAMA Dermatol. 2022 Oct 1;158(10):1131-1141. doi: 10.1001/jamadermatol.2022.2909. JAMA Dermatol. 2022. PMID: 35791876 Free PMC article.
-
Drug survival of adalimumab, ustekinumab and secukinumab in patients with psoriasis: a prospective cohort study from the British Association of Dermatologists Biologics and Immunomodulators Register (BADBIR).Br J Dermatol. 2020 Aug;183(2):294-302. doi: 10.1111/bjd.18981. Epub 2020 Mar 30. Br J Dermatol. 2020. PMID: 32124442
-
Treatment adherence and persistence of five commonly prescribed medications for moderate to severe psoriasis in a U.S. commercially insured population.J Dermatolog Treat. 2021 Sep;32(6):595-602. doi: 10.1080/09546634.2019.1687828. Epub 2020 Jan 8. J Dermatolog Treat. 2021. PMID: 31714168
-
Biologic Drug Survival in Psoriasis: A Systematic Review & Comparative Meta-Analysis.Front Med (Lausanne). 2021 Mar 18;7:625755. doi: 10.3389/fmed.2020.625755. eCollection 2020. Front Med (Lausanne). 2021. PMID: 33816514 Free PMC article.
-
Biologics for pediatric psoriasis: A systematic review and meta-analysis.Pediatr Dermatol. 2022 Jan;39(1):42-48. doi: 10.1111/pde.14870. Epub 2021 Dec 9. Pediatr Dermatol. 2022. PMID: 34888919
Cited by
-
Psoriasis Risk With Immune Checkpoint Inhibitors.JAMA Dermatol. 2025 Jan 1;161(1):31-38. doi: 10.1001/jamadermatol.2024.4129. JAMA Dermatol. 2025. PMID: 39504056
-
Genome‑wide association study and polygenic risk scores predict psoriasis and its shared phenotypes in Taiwan.Mol Med Rep. 2024 Jul;30(1):115. doi: 10.3892/mmr.2024.13239. Epub 2024 May 17. Mol Med Rep. 2024. PMID: 38757301 Free PMC article.
-
Drug Survival of IL-17 and IL-23 Inhibitors for Psoriasis: A Systematic Review and Meta-Analysis.Drugs. 2024 May;84(5):565-578. doi: 10.1007/s40265-024-02028-1. Epub 2024 Apr 17. Drugs. 2024. PMID: 38630365 Free PMC article.
-
Adherence, persistence and treatment switching in psoriasis.Immunotherapy. 2024;16(9):611-621. doi: 10.2217/imt-2023-0343. Epub 2024 Apr 23. Immunotherapy. 2024. PMID: 38651935 Free PMC article.
-
Incidence of HBV Reactivation in Psoriasis Patients Undergoing Cytokine Inhibitor Therapy: A Single-Center Study and Systematic Review with a Meta-Analysis.Viruses. 2024 Dec 30;17(1):42. doi: 10.3390/v17010042. Viruses. 2024. PMID: 39861831 Free PMC article.
References
-
- Chastek B., White J., Van Voorhis D., Tang D., Stolshek B. S. (2016). A Retrospective Cohort Study Comparing Utilization and Costs of Biologic Therapies and Jak Inhibitor Therapy across Four Common Inflammatory Indications in Adult Us Managed Care Patients. Adv. Ther. 33, 626–642. 10.1007/s12325-016-0312-y - DOI - PMC - PubMed
-
- Chiu H.-Y., Wang T.-S., Chen P.-H., Hsu S.-H., Tsai Y.-C., Tsai T.-F. (2018). Psoriasis in Taiwan: From Epidemiology to New Treatments. Dermatologica Sinica 36, 115–123. 10.1016/j.dsi.2018.06.001 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous