Maternal aromatase inhibition via letrozole altered RFamide-related peptide-3 and gonadotropin-releasing hormone expression in pubertal female rats
- PMID: 35656443
- PMCID: PMC9118272
- DOI: 10.22038/IJBMS.2022.60962.13499
Maternal aromatase inhibition via letrozole altered RFamide-related peptide-3 and gonadotropin-releasing hormone expression in pubertal female rats
Abstract
Objectives: Despite prevalence of polycystic ovary syndrome (PCOS) among childbearing women and development of many animal models for this syndrome, information on its etiology is still scarce. The intrauterine hyperandrogenic environment may underlie changes at the level of hypothalamus, pituitary, ovary organization in female offspring, and PCOS later in life. Letrozole has been shown to mimic reproductive and metabolic characteristics of PCOS in adult rodent models. Therefore, this research aimed to assess the condition in a prenatal letrozole-treated rat model.
Materials and methods: Twenty-eight female rats dams receiving letrozole at certain doses during late pregnancy were used in the trial. Pregnant Sprague-Dawley rats (n=21) received letrozole treatment on gestation days 16-18 at doses of 1.25, 1.0, 0.75, 0.5, and 0.25 mg/kg body weight (BW).
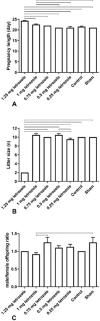
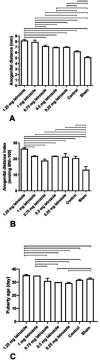
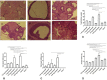
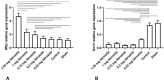
Results: Prenatal letrozole treatment delayed parturition time and reduced the litter size in pregnant dams (P<0.0001). Late puberty onset, irregular ovarian cyclicity, increased anogenital distance (AGD), body weight gain, serum testosterone concentration, and reduced estradiol levels (P<0.0001) were observed in the female offspring of dams receiving 1.25 and 1 mg/kg BW letrozole. Furthermore, letrozole at 1.25 and 1 mg/kg BW showed increased RFRP and decreased GnRH mRNA expression (P<0.0001). Letrozole treatment at doses of 1 mg/kg BW and lower was not fetotoxic.
Conclusion: It was concluded that 1 mg/kg BW letrozole may be suggested for prenatal PCOS induction.
Keywords: Gonadotropin-releasing – hormone; Hypothalamus; Letrozole; Polycystic ovary syndrome; Prenatal; RFamide-related peptide-3; Rat.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
The effects of letrozole-induced maternal hyperandrogenism on sexual behaviors, testicular histology, and serum biochemical traits in male offspring rats: An experimental study.Int J Reprod Biomed. 2023 Feb 8;21(1):71-82. doi: 10.18502/ijrm.v21i1.12669. eCollection 2023 Jan. Int J Reprod Biomed. 2023. PMID: 36875502 Free PMC article.
-
Letrozole-Induced Polycystic Ovary Syndrome Attenuates Cystathionine-β Synthase mRNA and Protein Abundance in the Ovaries of Female Sprague Dawley Rats.J Nutr. 2021 Jun 1;151(6):1407-1415. doi: 10.1093/jn/nxab038. J Nutr. 2021. PMID: 33758914 Free PMC article.
-
Partially purified non-polar phytocomponents from Aloe barbadensis Mill. gel restores metabolic and reproductive comorbidities in letrozole-induced polycystic ovary syndrome rodent model- an "in-vivo" study.J Ethnopharmacol. 2022 Jun 12;291:115161. doi: 10.1016/j.jep.2022.115161. Epub 2022 Mar 7. J Ethnopharmacol. 2022. PMID: 35271948
-
Pathophysiological changes in experimental polycystic ovary syndrome in female albino rats: Using either hemin or L-arginine.J Cell Physiol. 2019 Jun;234(6):8426-8435. doi: 10.1002/jcp.27757. Epub 2018 Nov 15. J Cell Physiol. 2019. PMID: 30443939 Review.
-
Kuntai Capsule Combined With Letrozole on Gonadal Hormone Levels and Ovarian Function in Patients With PCOS: A Systematic Review and Meta-Analysis.Front Endocrinol (Lausanne). 2021 Dec 28;12:789909. doi: 10.3389/fendo.2021.789909. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 35027910 Free PMC article.
Cited by
-
The effects of letrozole-induced maternal hyperandrogenism on sexual behaviors, testicular histology, and serum biochemical traits in male offspring rats: An experimental study.Int J Reprod Biomed. 2023 Feb 8;21(1):71-82. doi: 10.18502/ijrm.v21i1.12669. eCollection 2023 Jan. Int J Reprod Biomed. 2023. PMID: 36875502 Free PMC article.
References
-
- Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. 2018;14:270–284. - PubMed
-
- Krentowska A, Kowalska I. Metabolic syndrome and its components in different phenotypes of polycystic ovary syndrome. Diabetes Metab Res Rev. 2021:e3464. - PubMed
-
- Tamadon A, Hu W, Cui P, Ma T, Tong X, Zhang F, et al. How to choose the suitable animal model of polycystic ovary syndrome? Traditional Medicine and Modern Medicine. 2018;1:95–113.
-
- Abruzzese GA, Heber MF, Ferreira SR, Ferrer MJ, Motta AB. Prenatal androgen exposure affects ovarian lipid metabolism and steroid biosynthesis in rats. J Endocrinol. 2020;247:239–250. - PubMed
LinkOut - more resources
Full Text Sources
