The chemistry and biology of natural ribomimetics and related compounds
- PMID: 35656477
- PMCID: PMC9092360
- DOI: 10.1039/d2cb00019a
The chemistry and biology of natural ribomimetics and related compounds
Abstract
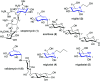
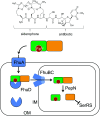
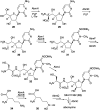
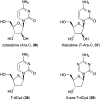
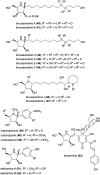
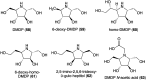
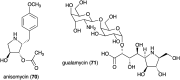
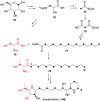
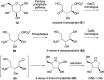
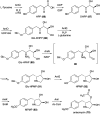
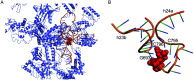
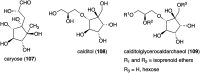
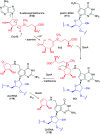
Natural ribomimetics represent an important group of specialized metabolites with significant biological activities. Many of the activities, e.g., inhibition of seryl-tRNA synthetases, glycosidases, or ribosomes, are manifestations of their structural resemblance to ribose or related sugars, which play roles in the structural, physiological, and/or reproductive functions of living organisms. Recent studies on the biosynthesis and biological activities of some natural ribomimetics have expanded our understanding on how they are made in nature and why they have great potential as pharmaceutically relevant products. This review article highlights the discovery, biological activities, biosynthesis, and development of this intriguing class of natural products.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




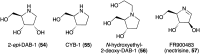

















References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

