Event-based modeling in temporal lobe epilepsy demonstrates progressive atrophy from cross-sectional data
- PMID: 35656586
- PMCID: PMC9540015
- DOI: 10.1111/epi.17316
Event-based modeling in temporal lobe epilepsy demonstrates progressive atrophy from cross-sectional data
Abstract
Objective: Recent work has shown that people with common epilepsies have characteristic patterns of cortical thinning, and that these changes may be progressive over time. Leveraging a large multicenter cross-sectional cohort, we investigated whether regional morphometric changes occur in a sequential manner, and whether these changes in people with mesial temporal lobe epilepsy and hippocampal sclerosis (MTLE-HS) correlate with clinical features.
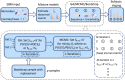
Methods: We extracted regional measures of cortical thickness, surface area, and subcortical brain volumes from T1-weighted (T1W) magnetic resonance imaging (MRI) scans collected by the ENIGMA-Epilepsy consortium, comprising 804 people with MTLE-HS and 1625 healthy controls from 25 centers. Features with a moderate case-control effect size (Cohen d ≥ .5) were used to train an event-based model (EBM), which estimates a sequence of disease-specific biomarker changes from cross-sectional data and assigns a biomarker-based fine-grained disease stage to individual patients. We tested for associations between EBM disease stage and duration of epilepsy, age at onset, and antiseizure medicine (ASM) resistance.
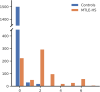
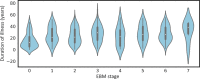
Results: In MTLE-HS, decrease in ipsilateral hippocampal volume along with increased asymmetry in hippocampal volume was followed by reduced thickness in neocortical regions, reduction in ipsilateral thalamus volume, and finally, increase in ipsilateral lateral ventricle volume. EBM stage was correlated with duration of illness (Spearman ρ = .293, p = 7.03 × 10-16 ), age at onset (ρ = -.18, p = 9.82 × 10-7 ), and ASM resistance (area under the curve = .59, p = .043, Mann-Whitney U test). However, associations were driven by cases assigned to EBM Stage 0, which represents MTLE-HS with mild or nondetectable abnormality on T1W MRI.
Significance: From cross-sectional MRI, we reconstructed a disease progression model that highlights a sequence of MRI changes that aligns with previous longitudinal studies. This model could be used to stage MTLE-HS subjects in other cohorts and help establish connections between imaging-based progression staging and clinical features.
Keywords: MTLE; disease progression; duration of illness; event-based model; patient staging.
© 2022 The Authors. Epilepsia published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
B.Ben. is cofounder of AIRAmed, a company that offers brain segmentation. C.D.W. is an employee of Biogen. D.J.S. has received research grants and/or consultancy honoraria from Lundbeck and Sun. K.H. has received honoraria and speaker fees from UCB, Eisai, and GW Pharma L.V. reports research funding from Biogen Australia, Life Molecular Imaging, and Eisai. N.K.F. has received honoraria from Arvelle, Bial, Eisai, Philips/EGI, and UCB. N.J. is MPI of a research grant from Biogen for work unrelated to the contents of this article. P.S. has received speaker fees and served on advisory boards for Biomarin, Zogenyx, GW and Pharmaceuticals; has received research funding from ENECTA, GW Pharmaceuticals, Kolfarma, and Eisai. P.M.T. has received a research grant from Biogen and was a paid consultant for Kairos Venture Capital for projects unrelated to this work. None of the other authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
- MR/M00841X/1/MRC_/Medical Research Council/United Kingdom
- R01 EB015611/EB/NIBIB NIH HHS/United States
- MR/K023152/1/MRC_/Medical Research Council/United Kingdom
- DH_/Department of Health/United Kingdom
- G0802012/MRC_/Medical Research Council/United Kingdom
- MR/K013998/1/MRC_/Medical Research Council/United Kingdom
- R01 NS065838/NS/NINDS NIH HHS/United States
- MR/L016311/1/MRC_/Medical Research Council/United Kingdom
- R01 MH117601/MH/NIMH NIH HHS/United States
- R01 AG059874/AG/NIA NIH HHS/United States
- R01 NS120976/NS/NINDS NIH HHS/United States
- S10 OD023696/OD/NIH HHS/United States
- MR/S03546X/1/MRC_/Medical Research Council/United Kingdom
- T32 MH018399/MH/NIMH NIH HHS/United States
- R01 MH116147/MH/NIMH NIH HHS/United States
- P41 EB015922/EB/NIBIB NIH HHS/United States
- MR/N026063/1/MRC_/Medical Research Council/United Kingdom
- MR/S00355X/1/MRC_/Medical Research Council/United Kingdom
- MR/T04294X/1/MRC_/Medical Research Council/United Kingdom
- R01 NS122827/NS/NINDS NIH HHS/United States
- R01 AG058854/AG/NIA NIH HHS/United States
- RF1 MH123163/MH/NIMH NIH HHS/United States
- R21 NS107739/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

